Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
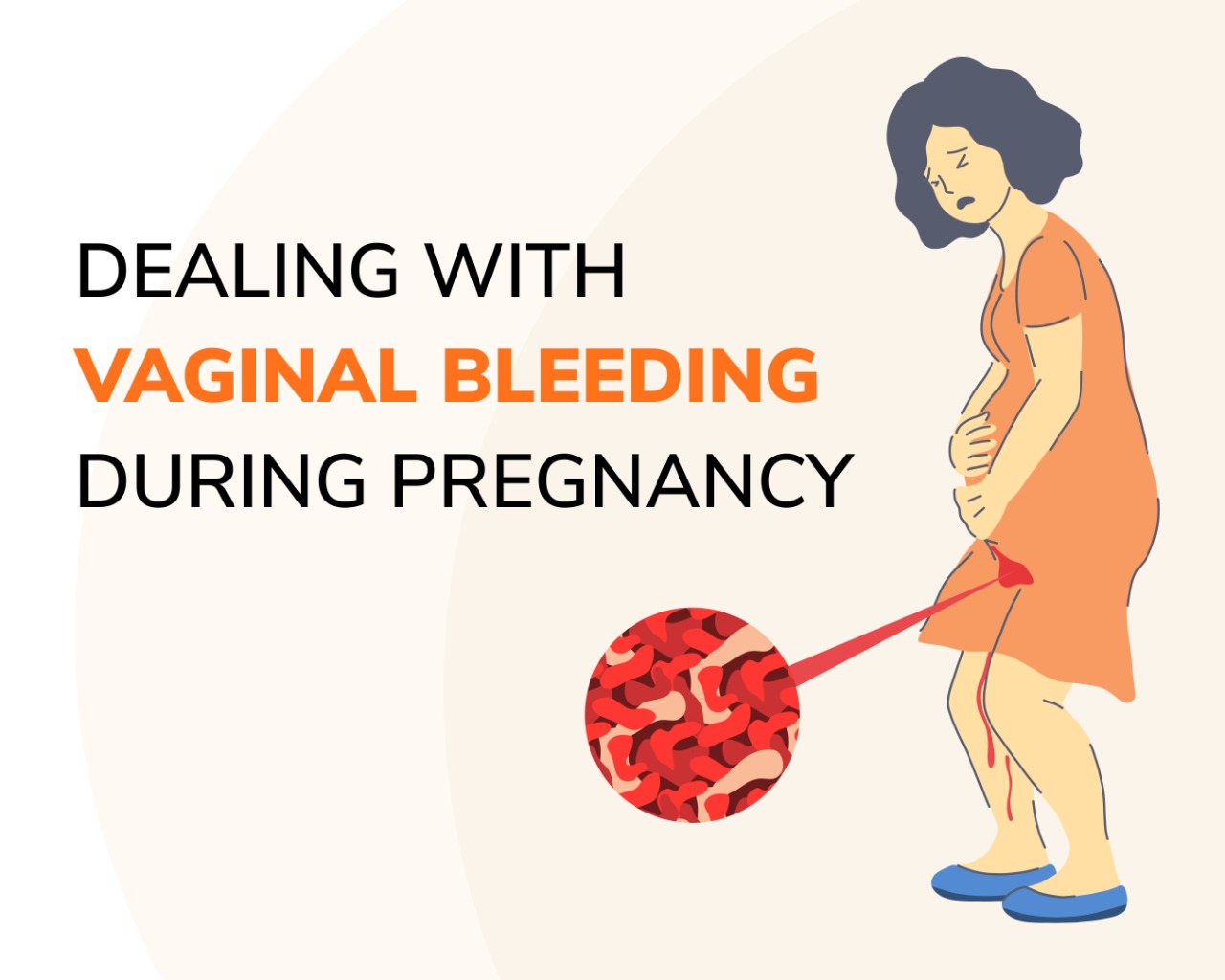
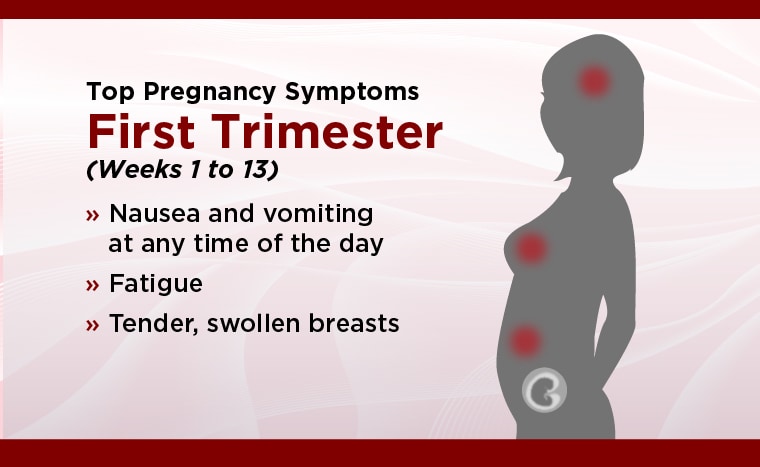
Pregnancy-related problems, such as preterm delivery (birth before week 37) or low birth weight (weighing less than 5 pounds, 8 ounces [2500 grams] at birth) have been reported in some studies looking at the use of gabapentin during pregnancy. In this review, we will summarize all available human data on the rate of infant MCMs associated with first trimester gabapentin monotherapy exposure as well as pregnancy outcomes, including rates of premature birth and low birth weight, in infants exposed to gabapentin at any time during gestation. We examined the risk of major congenital malformations and cardiac defects associated with gabapentin exposure during the first trimester (T1), and the risk of preeclampsia (PE), preterm birth (PTB), small for gestational age (SGA), and neonatal intensive care unit admission (NICUa) associated with gabapentin exposure early, late, or both early Gabapentin use in pregnancy is not very well-studied. While the available information does not strongly suggest that it causes problems for the baby, further research is required to prove that gabapentin is safe. The primary objective of this study was to examine the association between gabapentin treatment during the First trimester of pregnancy and all congenital malformations, major congenital malformations, and cardiac and orofacial malformations. Our objectives were to 1) determine whether first-trimester use of gabapentin is associated with an increased risk for major malformations; 2) examine rates of spontaneous abortions, therapeutic abortions, stillbirths, mean birth weight and gestational age at delivery; and 3) examine rates of poor neonatal adaptation syndrome following late In our study, only 28% of the women continued taking gabapentin throughout pregnancy as two-thirds of the women (66%) discontinued in the first trimester, most following pregnancy confirmation between 6 and 8 weeks’ gestation. Gabapentin exposures from 5 pregnancy registries, 1 HG pilot study and 2 additional cases were reviewed. Among 294 first trimester gabapentin-monotherapy exposures, there were 5 major congenital malformations (MCMs) reported (1.7%), which favorably compares to the MCM rate in the general population (1.6–2.2%). Pregnancy-related problems, such as preterm delivery (birth before week 37) or low birth weight (weighing less than 5 pounds, 8 ounces [2500 grams] at birth) have been reported in some studies looking at the use of gabapentin during pregnancy. We examined the risk of major congenital malformations and cardiac defects associated with gabapentin exposure during the first trimester (T1), and the risk of preeclampsia (PE), preterm birth (PTB), small for gestational age (SGA), and neonatal intensive care unit admission (NICUa) associated with gabapentin exposure early, late, or both early NSAIDS can be used during the first and second trimester, but there are differences between agents in terms of risk category. Ibuprofen, diclofenac, ketorolac and celecoxib can be considered safer options in cases NSAIDS are indicated. In general, short-term, episodic use of opioids appears to be safe in pregnancy. We examined the risk of major congenital malformations and cardiac defects associated with gabapentin exposure during the first trimester (T1), and the risk of preeclampsia (PE), preterm Gabapentin Pregnancy and Breastfeeding Warnings. Brand names: Fanatrex, Gabarone, Gralise, Neurontin. Pregnancy Warnings; Breastfeeding Warnings; Gabapentin Pregnancy Warnings. Benefits should clearly outweigh risks AU TGA pregnancy category: B3 US FDA pregnancy category: Not assigned Gabapentin exposures from 5 pregnancy registries, 1 HG pilot study and 2 additional cases were reviewed. Among 294 first trimester gabapentin-monotherapy exposures, there were 5 major congenital malformations (MCMs) reported (1.7%), which favorably compares to the MCM rate in the general population (1.6–2.2%). We examined the risk of major congenital malformations and cardiac defects associated with gabapentin exposure during the first trimester (T1), and the risk of preeclampsia (PE), preterm birth (PTB), small for gestational age (SGA), and neonatal intensive care unit admission (NICUa) associated with gabapentin exposure early, late, or both early We examined the risk of major congenital malformations and cardiac defects associated with gabapentin exposure during the first trimester (T1), and the risk of preeclampsia (PE), preterm birth (PTB), small for gestational age (SGA), and neonatal intensive care unit admission (NICUa) associated with gabapentin exposure early, late, or both early Pregnancy registry data have shown maternal first-trimester gabapentin monotherapy to be associated with a 1.2% rate of major congenital malformations among 659 infants, which compares favorably with the 1.6% to 2.2% major congenital malformation rate in the general population. Objectives: Our objectives were to 1) determine whether first-trimester use of gabapentin is associated with an increased risk for major malformations; 2) examine rates of spontaneous abortions, therapeutic abortions, stillbirths, mean birth weight and gestational age at delivery; and 3) examine rates of poor neonatal adaptation syndrome When treating neuropathic pain in a woman who is pregnant, the use of gabapentinoids (e.g. gabapentin) or an antiepileptic drug (AED) (e.g. levetiracetam, lamotrigine) is a last line option. This is due to the limited availability of data for safe use during pregnancy. Other options should be trialled first. Concerning exposure to gabapentin, increased risks of preterm birth, preeclampsia, small-for-gestational-age and NICU admission were reported in two studies.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |