Gallery
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 |  |
 | |
 |  |
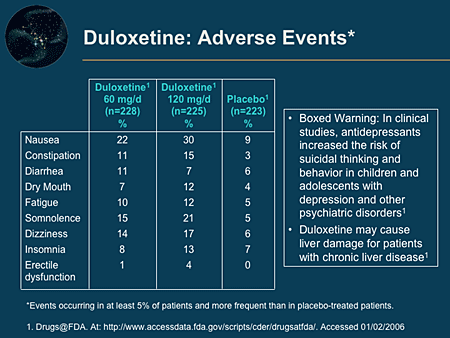
Study with Quizlet and memorize flashcards containing terms like Sarah, a 42-year-old female, requests a prescription for an anorexiant to treat her obesity. A trial of phentermine is prescribed. Prescribing precautions include: 1. Understanding that obesity is a contraindication to prescribing phentermine 2. Anorexiants may cause tolerance and should only be prescribed for 6 months 3 The new warnings may lead people to file drug lawsuits over breathing-related injuries blamed on gabapentin’s and pregabalin’s respiratory risks. Poison control centers have reported increased calls about the gabapentinoids. The FDA issued a warning in December 2019 that serious breathing difficulties may occur in patients using gabapentin (Neurontin, Gralise, Horizant) or pregabalin (Lyrica, Lyrica CR) who have respiratory risk factors.¹ The risk factors include the use of opioid analgesics and other drugs that depress the central nervous system (CNS) and Both gabapentin and pregabalin carry a warning statement for the risk of suicidal ideation and behavior. Since its approval in 1993, this association has been extensively research with gabapentin. Although few cases were reported in Phase III trials, the incidence of reports on suicidal ideation and behavior associated with the drug’s use has The Boxed Warnings added to pregabalin and gabapentin products advise prescribers to assess a patient's risk of misuse (for pregabalin), and abuse or dependence (for pregabalin and gabapentin) before prescribing these medicines, and to monitor them regularly during treatment. The U.S. Food and Drug Administration is warning that serious breathing problems can occur in patients who use gabapentin or pregabalin with opioids or other drugs that depress the central nervous system. Therapeutic and Goods Administration (TGA), Health authority of Australia released a box warning for medicines containing pregabalin and gabapentin for the risks of drug misuse, abuse, and dependence based on the continuous emerging safety data pertaining to these safety concerns. The agency is warning that serious breathing difficulties may occur in patients using gabapentin (Neurontin, Gralise, Horizant) or pregabalin (Lyrica, Lyrica CR) who have respiratory risk factors. Among those factors are use of opioid pain medicines and other drugs that depress the central nervous system (CNS), as well as conditions such as Boxed warnings, commonly referred to as 'black box' warnings, are issued by the U.S. Food and Drug Administration and featured in the labeling of drugs associated with serious adverse reactions. ISSUE: FDA is warning that serious breathing difficulties may occur in patients using gabapentin (Neurontin, Gralise, Horizant) or pregabalin (Lyrica, Lyrica CR) who have respiratory risk factors The U.S. Food and Drug Administration (FDA) is warning that serious breathing difficulties may occur in patients using gabapentin (Neurontin, Gralise, Horizant) or pregabalin (Lyrica, Lyrica CR FDA is warning that serious, life-threatening, and fatal respiratory depression has been reported with the gabapentinoids, gabapentin (Neurontin, Gralise, Horizant) and pregabalin (Lyrica, On December 19, 2019 FDA is warning that serious breathing difficulties may occur in patients using gabapentin (brand names Neurontin, Gralise, Horizant) or pregabalin (brand names Lyrica, Lyrica The United States Food and Drug Administration (FDA) has issued gabapentin warnings when this drug is taken with opioids. The reason is because when taken together, the drugs may cause respiratory problems. The FDA's warnings include the popular nerve drugs Neurontin, Gralise, Lyrica, and Horizant. The FDA recently released a warning for the medications, gabapentin (Neurontin, Gralise, Horizant) and pregabalin (Lyrica, Lyrica CR). The FDA warned that serious breathing difficulties may occur in patients using these medications who have respiratory risk factors. A black box warning – often referred to as simply a “boxed warning” – is the strongest warning issued by the FDA in the United States on drugs that carry specific health risks – serious or life-threatening adverse effects. The Food and Drug Administration is requiring that a new warning be added to the labeling of gabapentinoids concerning the risk of respiratory depression, especially when the drug is combined with other central nervous system (CNS) depressants or the patient has respiratory risk factors. Why you should pay attention to boxed drug warnings. Boxed medication warnings call attention to potentially serious or life-threatening side effects from using a prescription or over-the-counter A boxed warning is a mechanism used to highlight special warning statements in the Product Information (PI) to the prescriber and patient that could significantly alter the risk for patients when prescribed the product. Study with Quizlet and memorize flashcards containing terms like Lamotrigine has a black box warning about the possibility of which of the following? a. ototoxicity b. possible death c. liver toxicity d. fatal rashes, Patients with parkinson's disease have an imbalance of which of the following neurotransmitters? a. aspartate and serotonin b. GABA and glutamate c. glutamate and glycine d
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 |  |
 | |
 |  |