Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 | |
 | 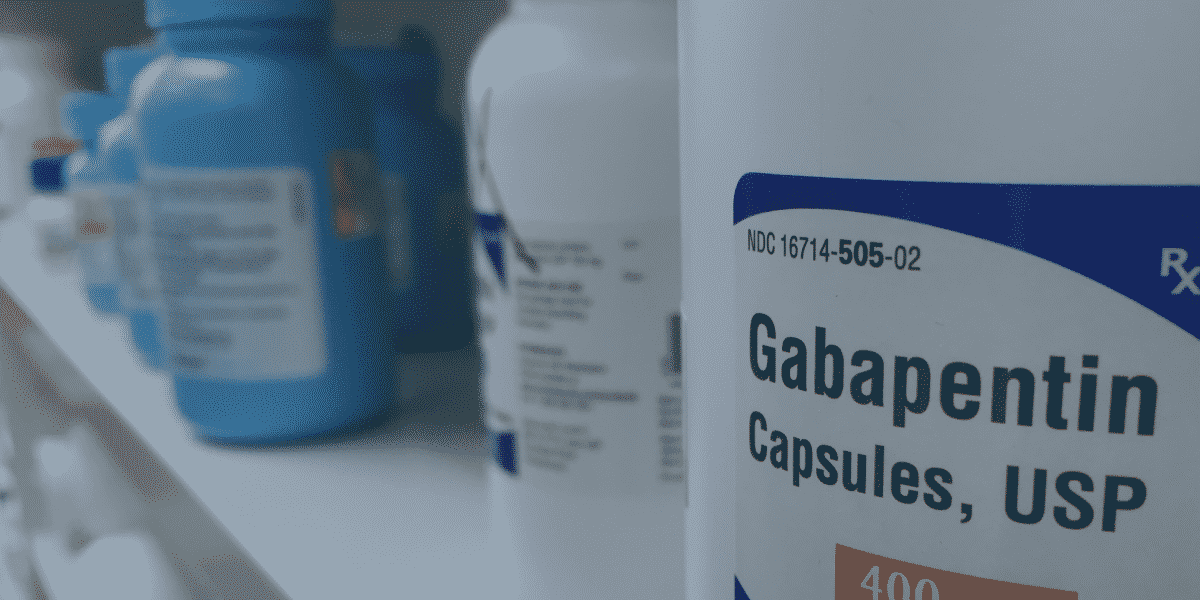 |
Update on Gabapentin in Ohio As a reminder, gabapentin is not considered a controlled substance in Ohio. The Board was made aware of incorrect communications made by a third-party vendor stating that Ohio had made gabapentin a controlled substance. While gabapentin is not a controlled substance, rule 4729:8-2-02 requires the following entities to Gabapentin isn’t a controlled substance according to the federal government. But several states have passed their own laws classifying gabapentin a schedule V (schedule 5) controlled substance. Combining gabapentin and opioids can be extremely dangerous. Discover the current status of gabapentin scheduling as a controlled substance across the US and the PDMP requirements for each state. Valuable insights for healthcare providers. , any new orders for Gabapentin issued by a practitioner WITHOUT a Utah. Controlled Substance license and a DEA registration will not be valid and MAY NOT be administered or dispensed. Prescription orders (including refills) issued for Gabapentin prior to May 1 , 2024, will not be. aected. It is not legal to distribute Gabapentin samples in Utah. The prevalence of gabapentin abuse has been estimated to be 1.6% in the general population compared with 15% to 22% in patients with opioid use disorders. 23 Individuals with a history of substance abuse (particularly opioids) or mental illness have been identified as having an increased risk of abusing gabapentin. 23 Evidence of gabapentin abuse was observed in a group of 503 individuals in Gabapentin isn’t a narcotic or federally controlled substance, but it is regulated and recognized as a controlled substance in certain states. Gabapentin is approved by the Food and 2. Is Gabapentin a controlled substance in North Carolina? • No, Gabapentin is not a controlled substance in North Carolina. 3. Why is Gabapentin included in the NC CSRS if it isn’t a controlled substance? • There is evidence that Gabapentin, when taken with opioids, can increase the risk of unintended overdose. January 9, 2019 – In an effort to continue to combat the opioid epidemic in Michigan, the Dept. of Licensing and Regulatory Affairs (LARA), with the support of the Michigan Board of Pharmacy, has modified its Pharmacy Rules to categorize Gabapentin as a Schedule 5 controlled substance. Gabapentin – or Neurontin – is a medication commonly Gabapentin is not currently listed as a controlled substance under the Controlled Substances Act of 1970. Individuals at the highest risk for abusing gabapentin include those with opioid abuse, mental illness, or previous history of prescription drug abuse. Gabapentin’s unscheduled status reflects its lower potential for abuse or dependency compared to controlled substances. However, the FDA monitors gabapentin for potential misuse, particularly when combined with other central nervous system depressants. Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). This commentary summarizes gabapentin's abuse potential, identifies state-level actions regarding gabapentin monitoring, and discusses possible clinical implications and ways to enhance patient safety when prescribing gabapentin. For controlled substance licensure, the rule changes require a designated prescriber to have a controlled substance license for a health facility if substances are stored there without an on-site pharmacy or an automated device stocked by a pharmacy, provide an exception to licensure for an emergency kit that contains controlled substances Gabapentin closely resembles pregabalin, a schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. The chemical structure of gabapentin is derived from the addition of a lipophilic cyclohexyl group to the backbone of gamma-aminobutyric acid (GABA). Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. Pregabalin is controlled in Schedule V of the Federal Controlled Substances Act . Although not controlled federally, some States also list gabapentin as a Schedule V controlled substance because of its abuse potential as a non-opioid pain reliever . iii At least 26 States and the District of Columbia have Gabapentin is approved to treat postherpetic neuralgia and epilepsy with partial-onset seizures. The large majority of gabapentin prescribing is off label. Gabapentin may be abused for euphoria, potentiating the high from opiates, reduction of alcohol cravings, a cocaine-like high, as well as sedation or sleep. Individuals at the highest risk for abusing gabapentin include those with opioid summarizes gabapentin’s abuse potential, identifies state-level actions regarding gabapentin monitoring, and discusses possible clinical implications and ways to enhance patient safety when prescribing gabapentin. Presently, seven states have classified gabapentin as a Schedule V controlled substance, and 12 others, New Jersey included, require that gabapentin prescriptions be reported in the PDMP system. Every time a prescription for gabapentin is filled out, it will automatically be added to the database. In the state of Kentucky, prescribers without a DEA license are unable to prescribe gabapentin after it was classified as a Schedule V controlled substance. 38 This licensing requirement is part of the state’s Controlled Substances Act which had the greatest impact on mid-level practitioners who may not have a DEA license. Kentucky
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 | |
 |  |