Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 | 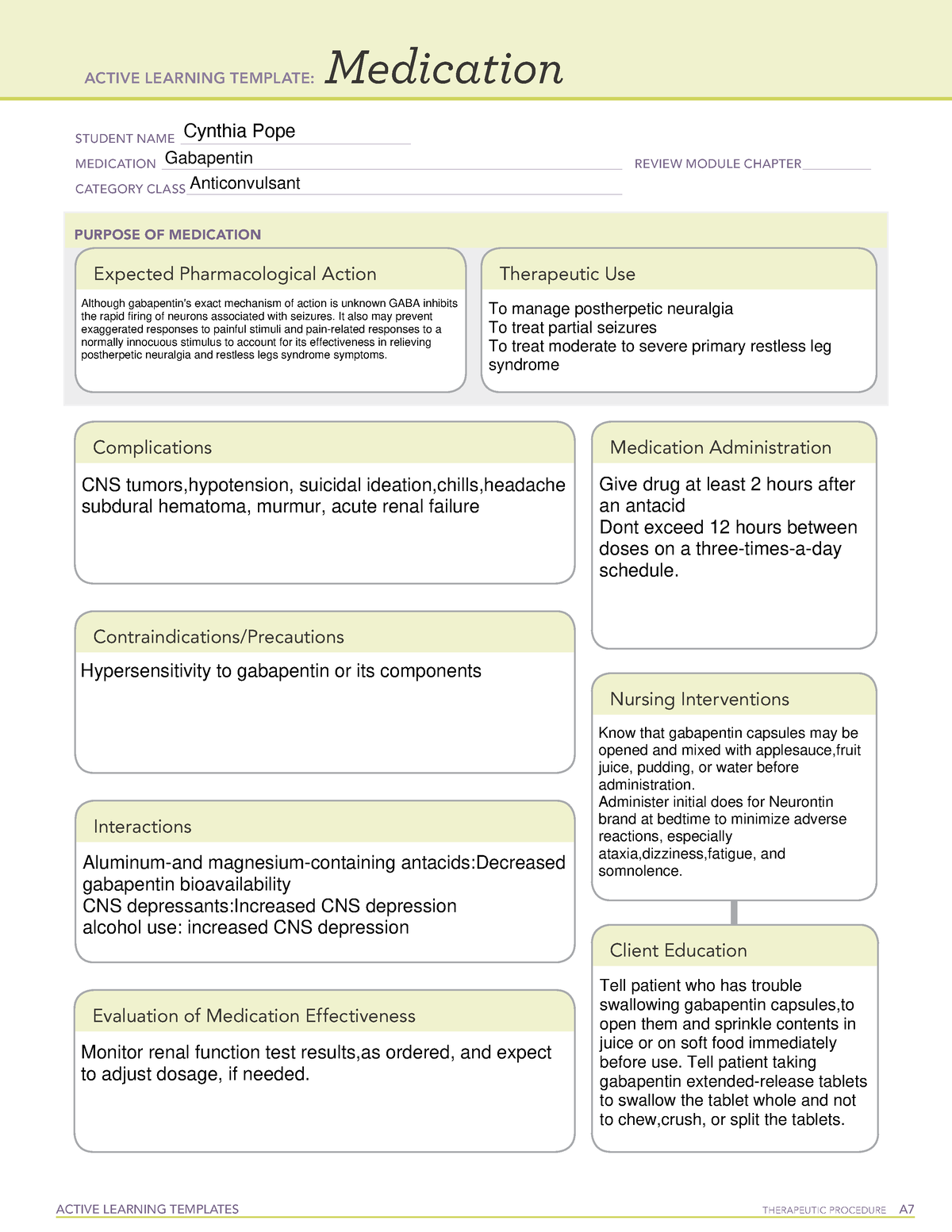 |
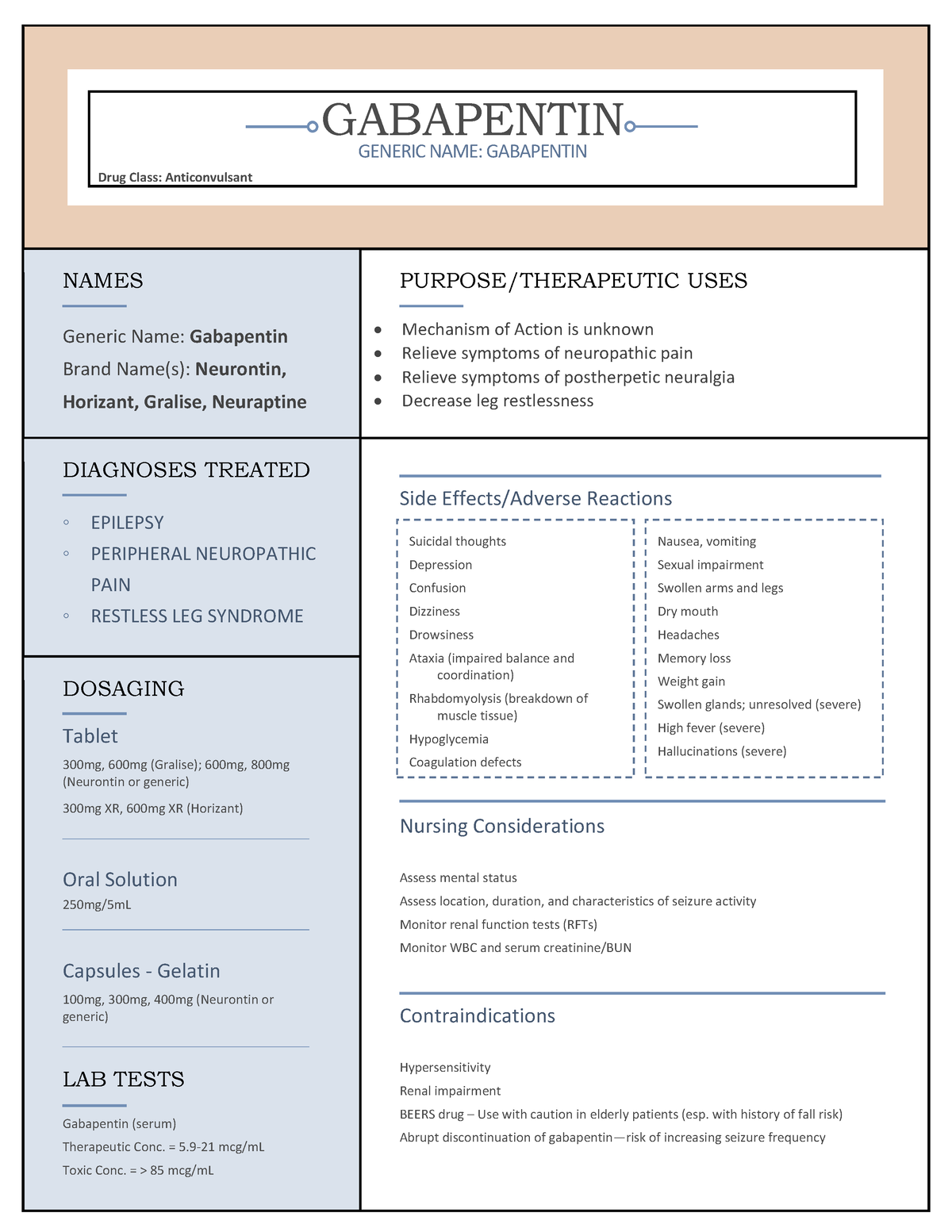
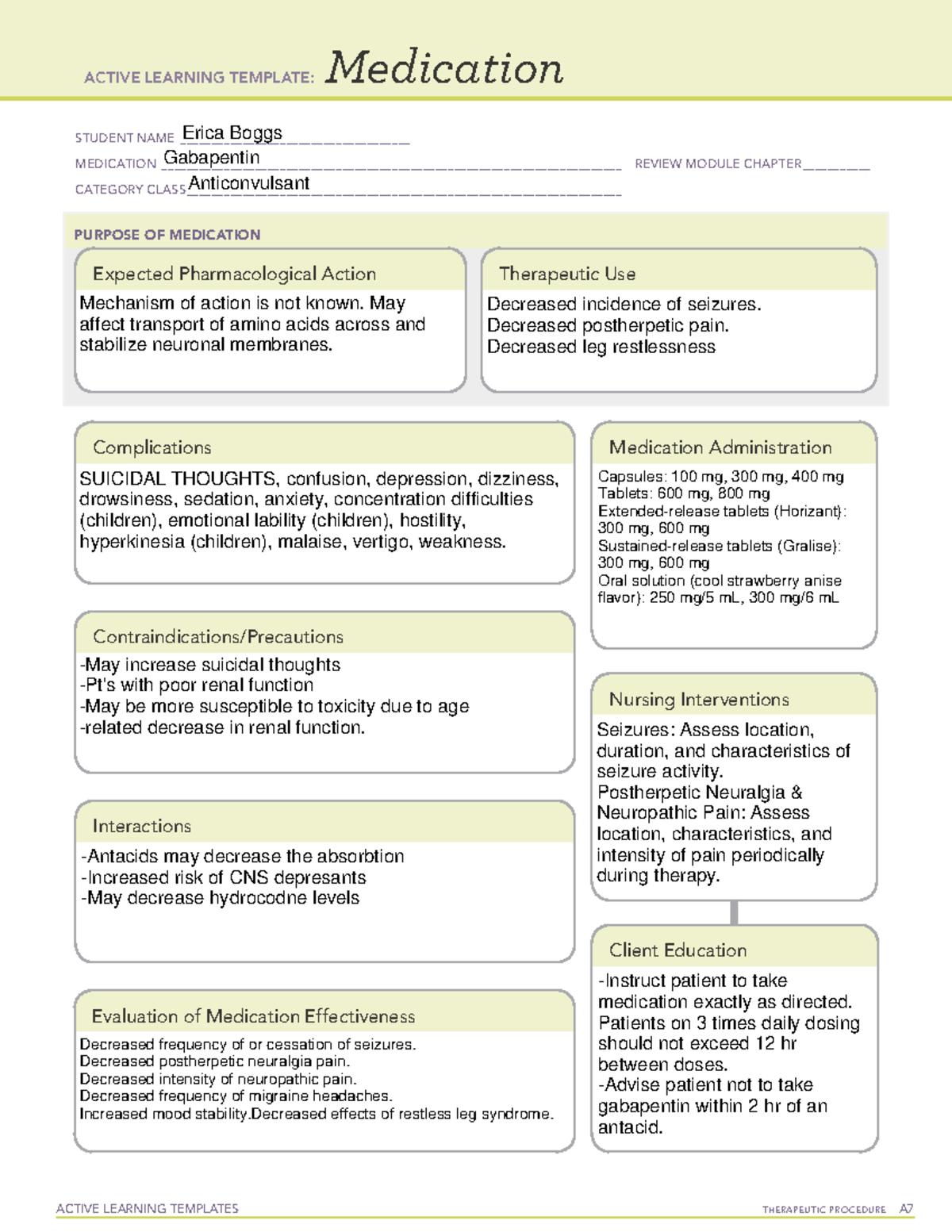
Appendix D of the Poisons and Therapeutic Goods Regulation 2008 (Regulation) lists Schedule 4 substances (prescription-only medicines) that have common therapeutic uses, but are also liable to abuse, misuse and diversion, warranting more stringent controls on possession and supply. As of 1 April 2019, pregabalin and gabapentin are controlled under the Misuse of Drugs Act 1971 as Class C substances and scheduled under the Misuse of Drugs Regulations 2001 as Schedule 3. Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Healthcare professionals should evaluate patients carefully for a history of drug abuse before prescribing gabapentin, and observe patients for signs of abuse and dependence. Gabapentin closely resembles pregabalin, a schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. The chemical structure of gabapentin is derived from the addition of a lipophilic cyclohexyl group to the backbone of GABA. But some states do control its use, labeling gabapentin as a Schedule 5 controlled substance. Why does gabapentin’s drug class vary from state to state? Although gabapentin isn’t controlled Table 2. Dosage Adjustments for Renal Impairment in Adults Receiving Gabapentin Gastroretentive Tablets60; Cl cr (mL/minute). Adjusted Dosage Regimen. 30–60. 600 mg to 1.8 g once daily; initiate at 300 mg once daily and may titrate according to same schedule recommended for those with normal renal function based on individual patient response and tolerability §60A-2-212. Schedule V. (a) Schedule V shall consist of the drugs and other substances, by whatever official name, common or usual name, chemical name, or brand name designated, listed in this section. States are now taking action to track gabapentin use through prescription monitoring programs, and some states have reclassified it as a Schedule V controlled substance. This commentary summarizes gabapentin's abuse potential, identifies state-level actions regarding gabapentin monitoring, and discusses possible clinical implications and ways some states have reclassified it as a Schedule V controlled substance. This commentary summarizes gabapentin’s abuse potential, identifies state-level actions regarding gabapentin monitoring, and discusses possible clinical implications and ways to enhance patient safety when prescribing gabapentin. © 2021 American Pharmacists Association But several states have passed their own laws classifying gabapentin a schedule V (schedule 5) controlled substance. Combining gabapentin and opioids can be extremely dangerous. Talk to your healthcare provider about the risks of gabapentin before taking it. Gabapentin is a prescription medication that falls into a class of drugs known as anticonvulsants, sometimes called anti-epileptic drugs. It works by reducing specific nerve signals sent to the brain, helping you cope with chronic pain and improve alertness. In seven states, gabapentin is classified as a schedule V controlled substance (including AL, KY, MI, ND, TN, VA, and WV). Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). Gabapentin is an anti-epileptic drug, also called an anticonvulsant. Drug class: Gamma-aminobutyric -Maximum time between doses in the 3 times a day schedule Gabapentin is not currently listed as a controlled substance under federal law. However, some states classify gabapentin as a Schedule V substance or a drug of concern and mandate reporting to PMP. Furthermore, other states are considering similar actions due to increasing evidence of associated risks. Annual Review Completed for all Drug Entries on 9-15-2022 . Please be advised that the information contained in this table is compiled solely from reference works recognized and approved by the State Board of Pharmacy pursuant to rule 4729:9-2-01. Abbreviations Used in Reference Table C.S.A Schedules I Schedule I II Schedule II III Schedule III Effective 1 July 2017, Kentucky classified gabapentin as a schedule V controlled substance statewide. [126] Gabapentin is scheduled V drug in other states such as West Virginia, [127] Tennessee, [128] Alabama, [129] Utah, [130] and Virginia. [131] Neurontin; Ther. Class. analgesics. anticonvulsants. mood stabilizers. Pharm. Class. gamma aminobutyric acid gaba analogues. Controlled Substance Schedule: V(only schedule V in some states) There's more to see -- the rest of this topic is available only to subscribers. Gabapentin is not a narcotic or federally controlled substance, but it is classified as a Schedule V drug in certain states due to its potential for abuse and diversion. Learn which states control gabapentin, why it is regulated, and how it can interact with opioids and other drugs. Drug Schedules Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules depending upon the drug’s acceptable medical use and the drug’s abuse or dependency potential. The abuse rate is a determinate factor in the scheduling of the drug; for example, Schedule I drugs have a high potential for abuse and the potential to create Schedule III in KY (902 KAR 55:015). Schedule IV federally. Phenobarbital & noncontrolled active ingredient; 2285 III; N Quadrapax, Phenohydro, PB Hyos elixir; Schedule III in KY (schedule IV federally). Some products with no significant potential for abuse ; specifically exempted (see 902 KAR 55:045/CFR 1308.32). Link to current list
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |