Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
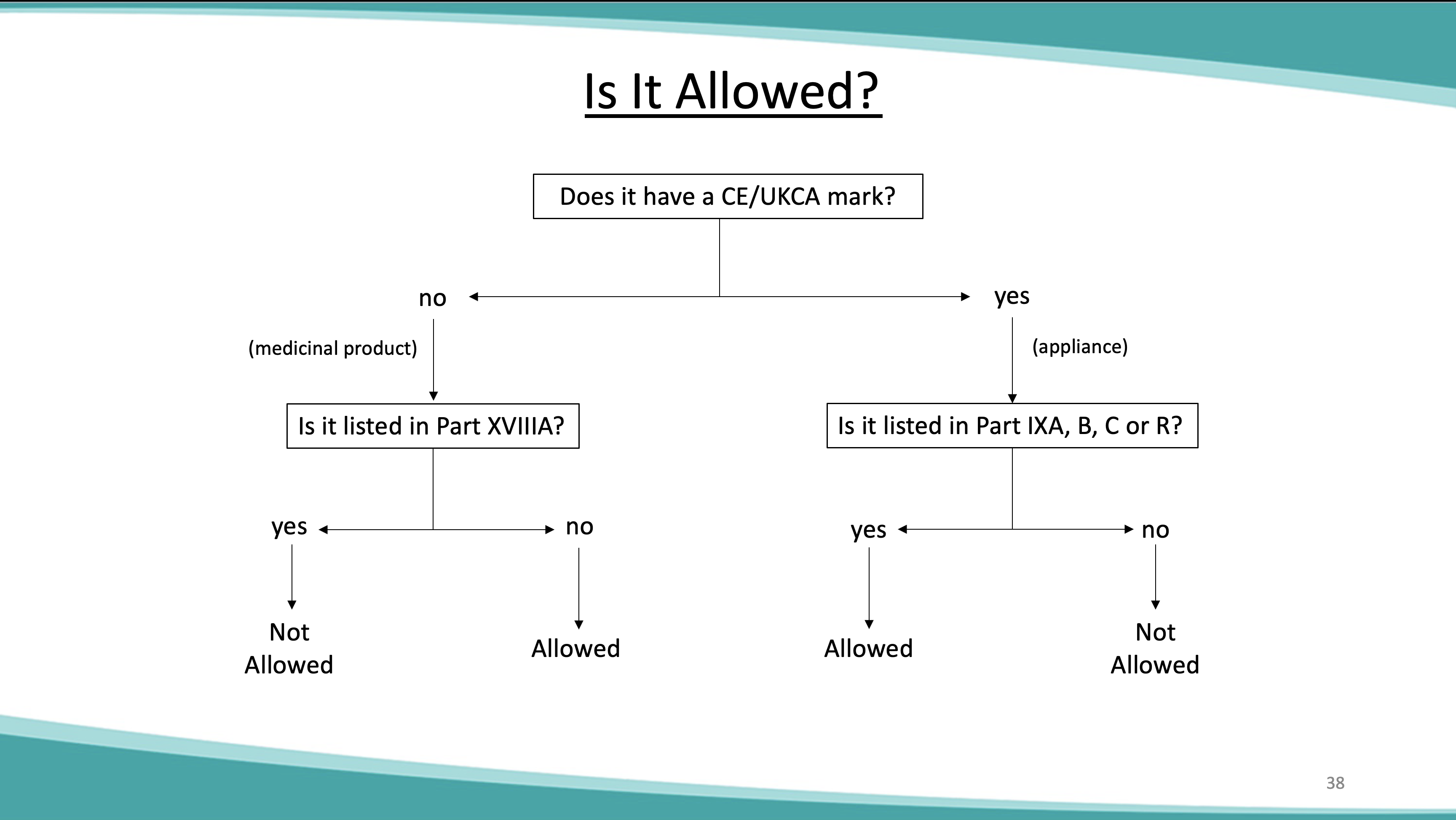
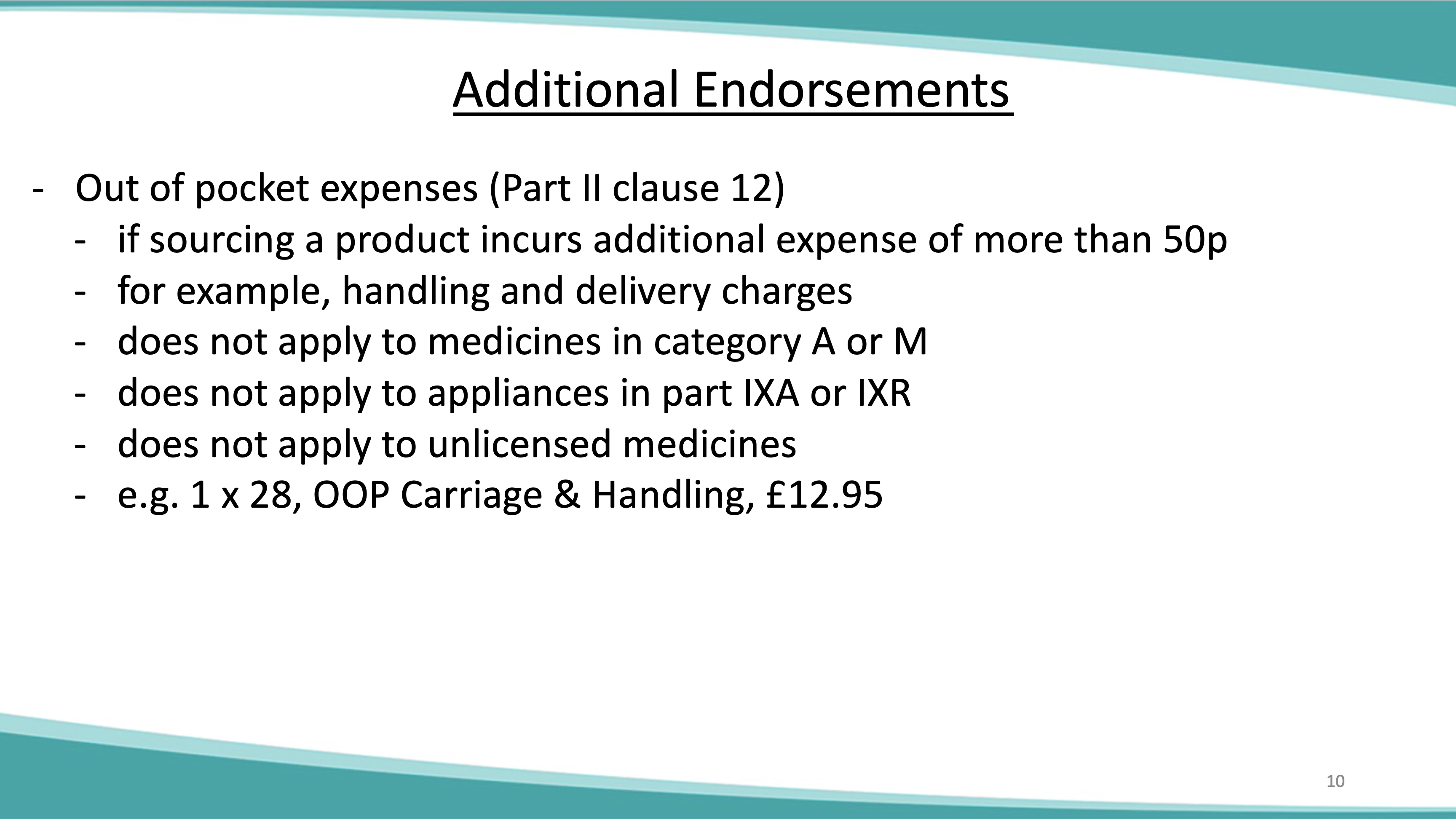
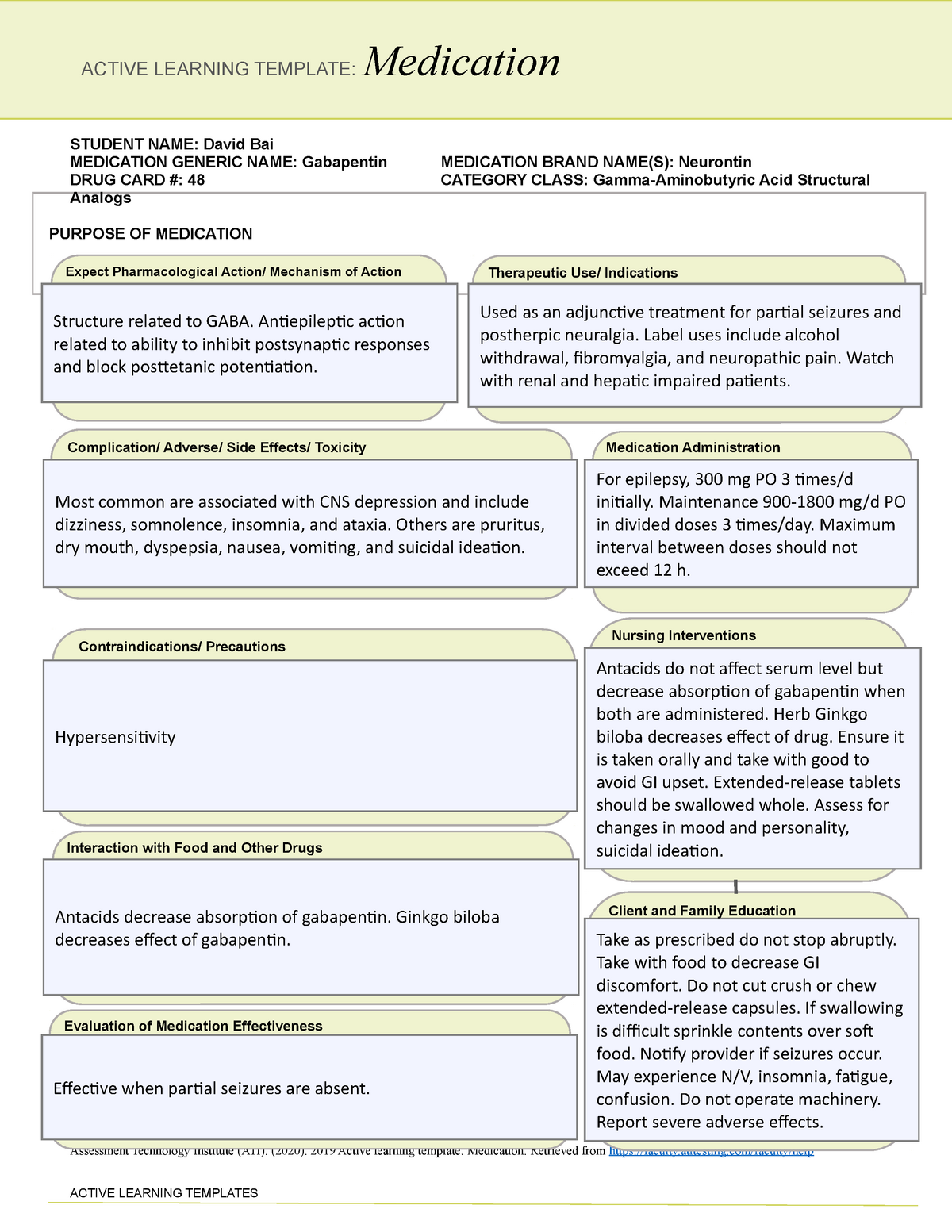
Dispensing contractors should note that from April 2021 the Drug Tariff will no longer be printed and distributed. March 2021 will be the final month in which a printed Drug Tariff will be circulated. The Drug Tariff will continue to be published on a monthly basis and accessible at the following website link: NHS Prescription Services produces the Drug Tariff on a monthly basis on behalf of the Department of Health and Social Care. It’s supplied primarily to pharmacists and GP practices. The Drug Tariff outlines: You can view the Drug Tariff 3 working days before the 1 st of each month. The published prices for these Part 1 products will continue to be shown both in the NHS dictionary of medicines and devices (dm+d) and the Northern Ireland Drug Tariff. Concessionary prices are set by DH England following discussions with Community Pharmacy England (formerly PSNC). Check to see if there are any updates to the published Drug Tariff each month. Drug Tariff payment category Part VIIIA Category A. Drug Tariff Price Start Date End Date; Current Price 3844 : Start Date 01-10-2024 : End Date: Previous Price 5117 Gabapentin 600mg tablets £9.75; Isosorbide mononitrate 10mg tablets £5.00; and not as printed in the August edition of the Drug Tariff. Pregabalin and gabapentin reclassification Here we explain the prescribing and dispensing changes that will need to take place when pregabalin and gabapentin become Schedule 3 Controlled Drugs. Summary Requirements for gabapentin and pregabalin from 1st April 2019 are as follows: Controlled Drug Prescription requirements Prescription validity In your letter dated December 7, 2011 you requested a tariff classification ruling. The subject product, Gabapentin Tablets, is a medicinal preparation containing, Gabapentin, as the active ingredient. It is indicated for the treatment of seizures and neuropathic pains. Gabapentin: Based on Ingredient Substance: 600 mg: Name. Drug Tariff Price Drug Tariff Payment Category; 10 tablet: 100 tablet: 541: Part VIIIA Category M NHS Electronic Drug Tariff uses cookies. Some of the cookies we use are essential for parts of the site to operate and have already been set. Other cookies may be set to remember your preferences. By continuing to use NHS Electronic Drug Tariff you are agreeing that this is acceptable to you. More details can be found in our %PDF-1.6 %âãÏÓ 550 0 obj > endobj 566 0 obj >/Filter/FlateDecode/ID[]/Index[550 28]/Info 549 0 R/Length 87/Prev 520694/Root 551 0 R/Size 578/Type/XRef/W[1 3 1 Advanced Service Specification – NHS New Medicine Service (NMS) [PDF: 245KB]As outlined in Part VIC of the drug tariff, you should only prescribe NMS to patients who’ve been prescribed a particular NMS medicine for the first time and for the following conditions. You can view each condition and its drug list, last updated September 2021. Start typing to search for tariff data about a particular generic presentation: NHS Drug Tariff price of Gabapentin 600mg tablets for March 2025. See if it qualifies as a special container, has a concession price, or if a discount is not deducted. Drug Tariff Pro brings you the most comprehensive drug tariff information. When community pharmacies cannot source a drug at or below the reimbursement price as set out in the Drug Tariff, the Department of Health and Social Care (DHSC) can introduce a price concession at the request of Community Pharmacy England. Changes to Category M reimbursement prices in Part VIIIA of the Drug Tariff. Category M for January 2025 contains adjustments for underlying market prices (between July – Sep 2024) and the latest results of the Medicine Margin Survey (up until end of June 2024). The Department of Health has agreed that the September Drug Tariff Part VIIIA reimbursement prices for the following items will be: Amitriptyline 50mg tablets £3.44 Bumetanide 1mg tablets £2.20 Start typing to search for tariff data about a particular generic presentation: pregabalin and gabapentin will be included in the list of “exempted drugs” in the safe custody regulations which means that community pharmacy teams will not be required to keep these drugs in the CDs cabinet. Requirements for gabapentin and pregabalin from 1st April 2019 are as follows: Controlled Drug Prescription requirements Prescription Forms available from special-order manufacturers include: oral suspension, oral solution. Warning: This medicine may make you sleepy. If this happens, do not drive or use tools or machines. Rhybudd: Gall y feddyginiaeth hon eich gwneud yn gysglyd. Peidiwch â gyrru, defnyddio offer llaw neu beiriannau os yw hyn yn digwydd.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |