Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
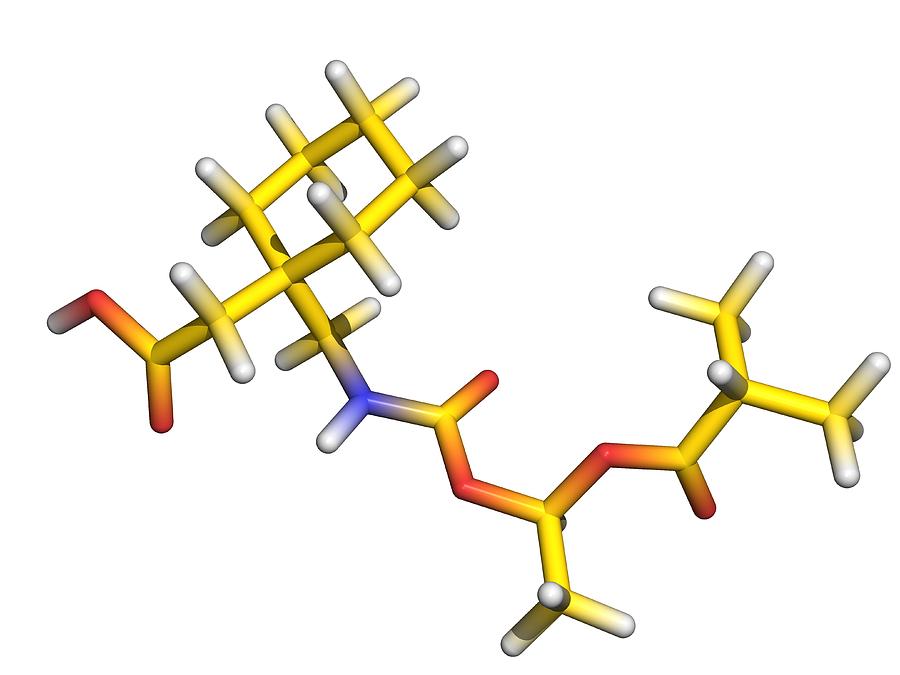 |  |
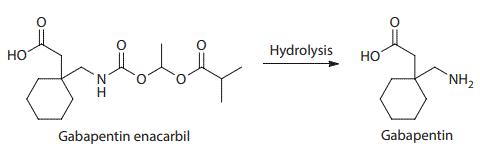
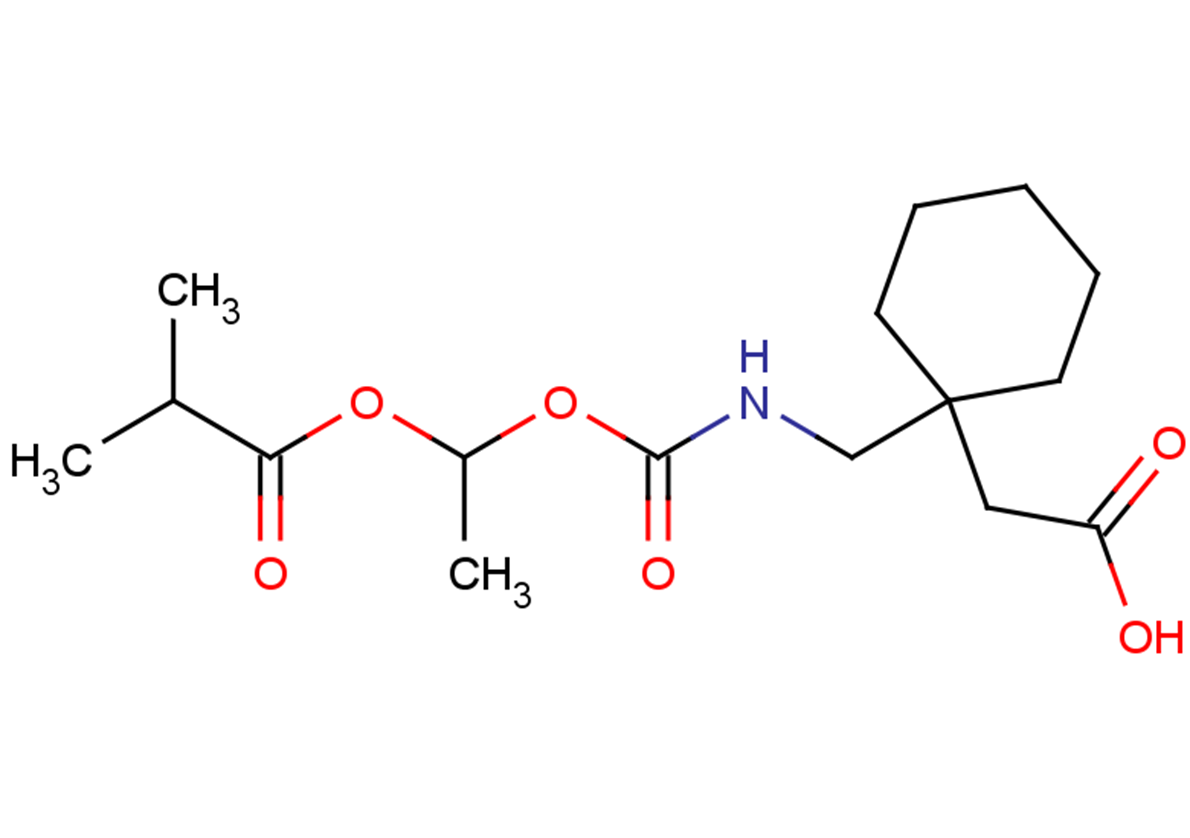
gabapentin enacarbil: Xenoport: moderate-to-severe restless legs syndrome (RLS) 4/6/2011: 28:12.92 - Anticonvulsants, Misc; 28:08.92 - Analgesics and Antipyretics, Misc : Zytiga ® abiraterone acetate: Centocor Ortho Biotech: metastatic castration-resistant prostate cancer: 4/28/2011: 10:00 - Antineoplastic Agents : Trajenta ® linagliptin GABAPENTIN ENACARBIL is a small molecule drug with a maximum clinical trial phase of IV (across all indications) that was first approved in 2011 and is indicated for neuralgia and restless legs syndrome and has 4 investigational indications. See also: Gabapentin (has active moiety); Gabapentin encarbil (annotation moved to). Horizant (gabapentin enacarbil) is used to treat restless legs syndrome and nerve pain caused by the herpes virus. Includes Horizant side effects, interactions and indications. Gabapentin Enacarbil has severe interactions with no other drugs. Gabapentin Enacarbil has serious interactions with the following drugs: metoclopramide intranasal; ropeginterferon alfa 2b; Gabapentin Enacarbil has moderate interactions with at least 127 other drugs. Gabapentin Enacarbil has minor interactions with at least 17 other drugs. VET: In general, avoid the use of the commercially available human oral solution (Neurontin) in dogs as it reportedly contains 300 mg/mL xylitol.As the threshold dose that can cause hypoglycemia in dogs is approximately 100 mg/kg doses of up to 15 mg/kg in dogs using the solution should be safe, but further data is needed to confirm this Additionally, xylitol may be hepatotoxic in dogs. HORIZANT® (gabapentin enacarbil) Extended-Release Tablets are indicated for the treatment of moderate-to-severe primary Restless Legs Syndrome (RLS) in adults. HORIZANT is not recommended for D09539 Gabapentin enacarbil (JAN/USAN/INN) <JP/US> New drug approvals in the USA [ br08319.html ] New molecular entities and new therapeutic biological products Horizant (gabapentin enacarbil) is an extended release tablet used to treat restless legs syndrome and for the pain from having shingles (postherpetic nerve pain). Generic brands of gabapentin capsules, USP are used for postherpetic nerve pain and for add on therapy for partial onset seizures in patients 3 years and older Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Healthcare professionals should evaluate patients carefully for a history of drug abuse before prescribing gabapentin, and observe patients for signs of abuse and dependence. Gabapentin enacarbil (Horizant (ER) (U.S. Tooltip United States), Regnite (in Japan)) is an anticonvulsant and analgesic drug of the gabapentinoid class, and a prodrug to gabapentin. [1] Gabapentin was first conceptualised in the early 1970s during efforts to discover drugs for treating neurological disorders. 7 Gamma-aminobutyric acid (GABA) was known to be a key inhibitory neurotransmitter, whose inhibition could cause seizures. Compare Gabapentin vs Gabapentin Enacarbil head-to-head with other drugs for uses, ratings, cost, side effects and interactions. Gabapentin enacarbil, (R)- | C16H27NO6 | CID 66864580 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. Table 2. Dosage Adjustments for Renal Impairment in Adults Receiving Gabapentin Gastroretentive Tablets60; Cl cr (mL/minute). Adjusted Dosage Regimen. 30–60. 600 mg to 1.8 g once daily; initiate at 300 mg once daily and may titrate according to same schedule recommended for those with normal renal function based on individual patient response and tolerability Horizant® is the only medication in its class that is FDA approved to treat RLS. 5-7 Learn more about this once-daily treatment option (gabapentin enacarbil) is Generic Name Gabapentin DrugBank Accession Number DB00996 Background. Gabapentin is a structural analogue of the inhibitory neurotransmitter gamma-aminobutyric acid that was first approved for use in the United States in 1993. 16 It was originally developed as a novel anti-epileptic for the treatment of certain types of seizures 14,5 - today it is also widely used to treat neuropathic pain. 8 Gabapentin enacarbil may decrease the excretion rate of Synthetic Conjugated Estrogens, A which could result in a higher serum level. Synthetic Conjugated Estrogens, B: Gabapentin enacarbil may decrease the excretion rate of Synthetic Conjugated Estrogens, B which could result in a higher serum level. Tacrolimus Gabapentin Enacarbil converts to gabapentin rapidly by non-specific carboxylesterase primarily in enterocytes and to a lesser extent in the liver upon absorption. The concentration of intact prodrug in blood is transient and ≤ 2% of the corresponding gabapentin level. SOLZIRA™ is proposed to be marketed as 600 mg ER Tablets Gabapentin enacarbil is classified as a BCS Class 2 compound (low solubility, high permeability). The applicant provided adequate information regarding structure elucidation and confirmation, Therapeutic Class: Analgesic. Pharmacologic Class: Gabapentinoid. Chemical Class: Gamma Aminobutyric Acid (class)
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |