Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
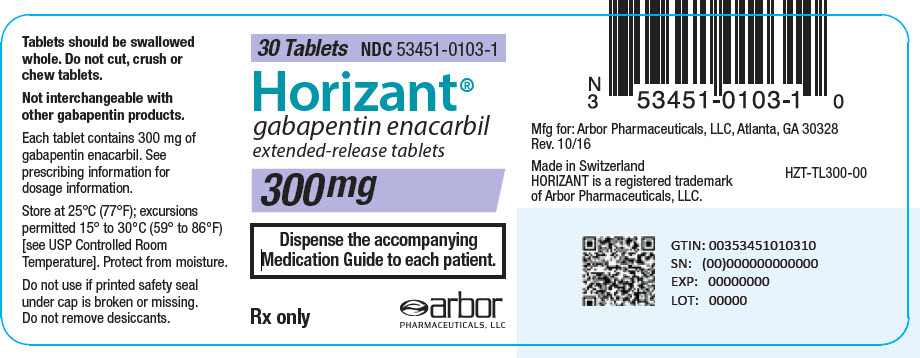
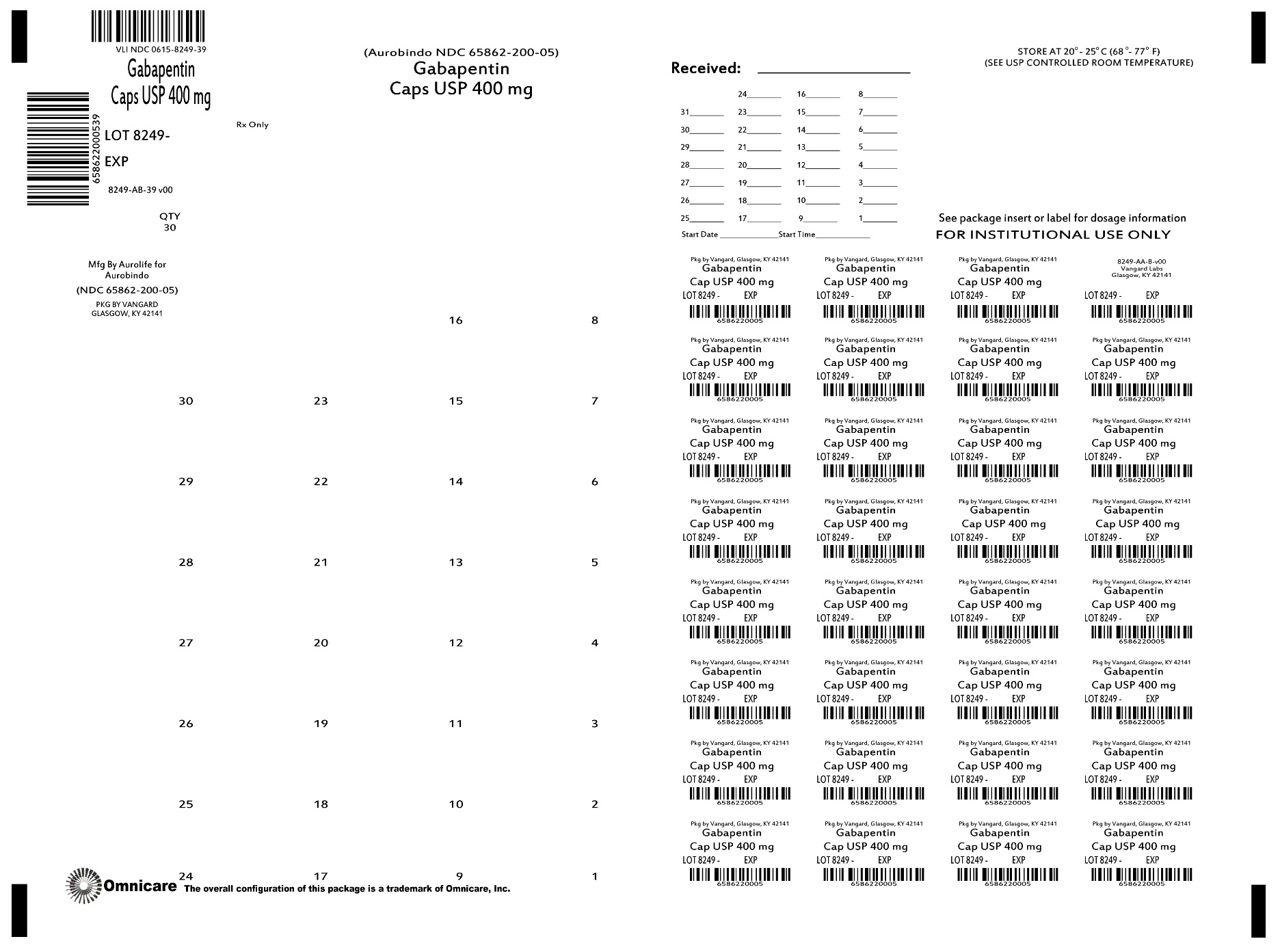
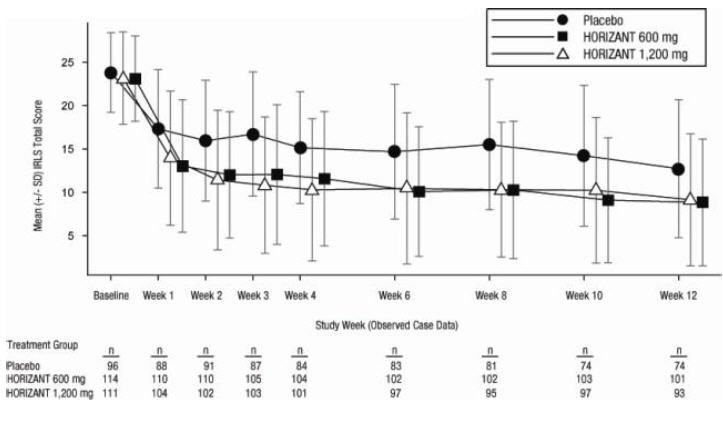
Gababentin Enacarbil (GEn) is a prodrug of gabapentin. Gabapentin, the active metabolite of GEn, is FDA approved for use as an anticonvulsant and for pain relief in postherpetic neuralgia. Detailed Gabapentin Enacarbil dosage information for adults and children. Includes dosages for Restless Legs Syndrome, Epilepsy and Postherpetic Neuralgia; plus renal, liver and dialysis adjustments. Horizant (gabapentin enacarbil) Extended-Release Tablets Company: GlaxoSmithKline Application No.: 022399 Approval Date: 4/06/2011. Persons with disabilities having problems accessing the PDF HORIZANT (gabapentin enacarbil) Extended-Release Tablets for oral use This label may not be the latest approved by FDA. For current labeling information, please Senior Specialist, Regulatory Affairs, Labeling 6 Concourse Parkway Atlanta, GA 30328 . Dear Ms. Portik: Please refer to your supplemental new drug application (sNDA) dated and received January 17, 2020, submitted under section 505(b) of the Federal Food, Drug, and Cosmetic Act (FDCA) for Horizant (gabapentin enacarbil). Drug Substance: Gabapentin enacarbil (also called XP 13512) is a non-ester, pro-drug of gabapentin (a marketed drug). It has a molecular formula C16H27NO6 and molecular weight 329.39. There is a single chiral center , the drug substance is a racemic mixture. Gabapentin enacarbil is a white to off-white powder, soluble in (b) (4) (b) (4) (b) (4) NDA 022399 - FDA APPROVED LABELING (March 2013) 1 HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use HORIZANT safely and effectively. See full prescribing information for HORIZANT. HORIZANT (gabapentin enacarbil) Extended-Release Tablets for oral use. Initial U.S. Approval: 2011 HORIZANT (gabapentin enacarbil) Extended-Release Tablets are indicated for the management of postherpetic neuralgia (PHN) in adults. 2 DOSAGE AND ADMINISTRATION . Tablets should be swallowed The US Food and Drug Administration (FDA) has approved gabapentin enacarbil (Horizant) extended-release tablets for the management of postherpetic neuralgia in adults. The drug, approved in April 2011 for the treatment of restless legs syndrome (RLS), is now approved to treat PHN at a dose of 600 mg twice daily, notes a joint statement from Gabapentin enacarbil was approved by the Food and Drug Administration (FDA) in April of 2011. This article reviews clinically significant aspects of this new drug including: the FDA-approved indications, mechanism of action, administration, drug interactions, adverse effects, clinical trial evidence, innovative properties and place in therapy. Gabapentin enacarbil extended release tablets available under the trade name Horizant and gabapentin are not interchangeable. Use: Postherpetic neuralgia. Usual Adult Dose of Horizant for Restless Legs Syndrome: 600 mg orally once daily with food at about 5 PM. Horizant ® is the only FDA-approved extended-release prodrug of gabapentin for Postherpetic Neuralgia (PHN) 1,2. Horizant ® offers single-step titration and reaches therapeutic dose within 4 days 1. No titration is required. Patients should take once daily at about 5 PM 1. Tablets should be swallowed whole and should not be cut, crushed, or chewed. HORIZANT® (gabapentin enacarbil) Extended-Release Tablets are indicated for the treatment of moderate-to-severe primary Restless Legs Syndrome (RLS) in adults. HORIZANT is not recommended for Take Horizant exactly as prescribed by your doctor. Follow all directions on your prescription label. Do not take this medicine in larger or smaller amounts or for longer than recommended. Do not cut, crush, chew, or break an extended-release Horizant tablet. Swallow the tablet whole. Background: The FDA evaluated drug use, which showed an increased prescription usage of gabapentinoids. Between 2012 and 2016, the FDA estimated the number of patients who filled a gabapentin prescription increased from 8.3 million to 13.1 million annually. In late December 2019, the U.S. Food and Drug Administration (FDA) announced that it will require new warning labels for gabapentinoids. These labels will address the risk of serious respiratory distress leading to death in patients who combine the treatment with an opioid. There is limited information regarding Postmarketing Experience of Gabapentin Enacarbil in the drug label. Drug Interactions. Gabapentin enacarbil is released faster from HORIZANT Extended-Release tablets in the presence of alcohol. Consumption of alcohol is not recommended when taking HORIZANT Gabapentin enacarbil is a gabapentin prodrug used to treat Restless Legs Syndrome (RLS) From FDA label. water solubility: Solubility of 0.5 mg/mL in water : Gabapentin enacarbil (Horizant™) extended release tablets received FDA approval in April 2011 for the treatment of moderate-to-severe primary Restless Legs Syndrome in adults. 1 Gabapentin immediate release (Neurontin) was first approved by the FDA in 1994 for the adjunct treatment of partial seizures and is also FDA approved for the treatment o References: Horizant [package insert] Woburn, MA: Azurity Pharmaceuticals, Inc.; 2022 ; Food and Drug Administration. Orange Book: approved drug products with therapeutic equivalence evaluations [gabapentin enacarbil].
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |