Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
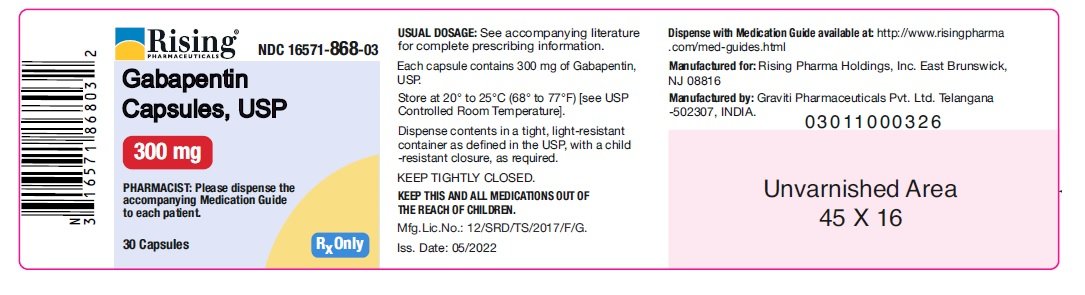
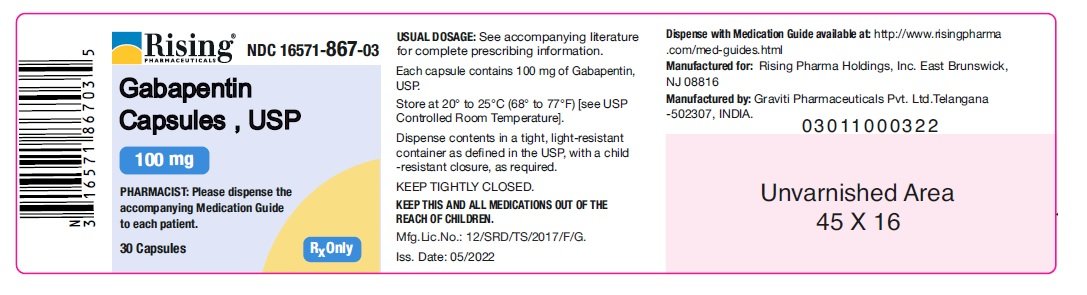
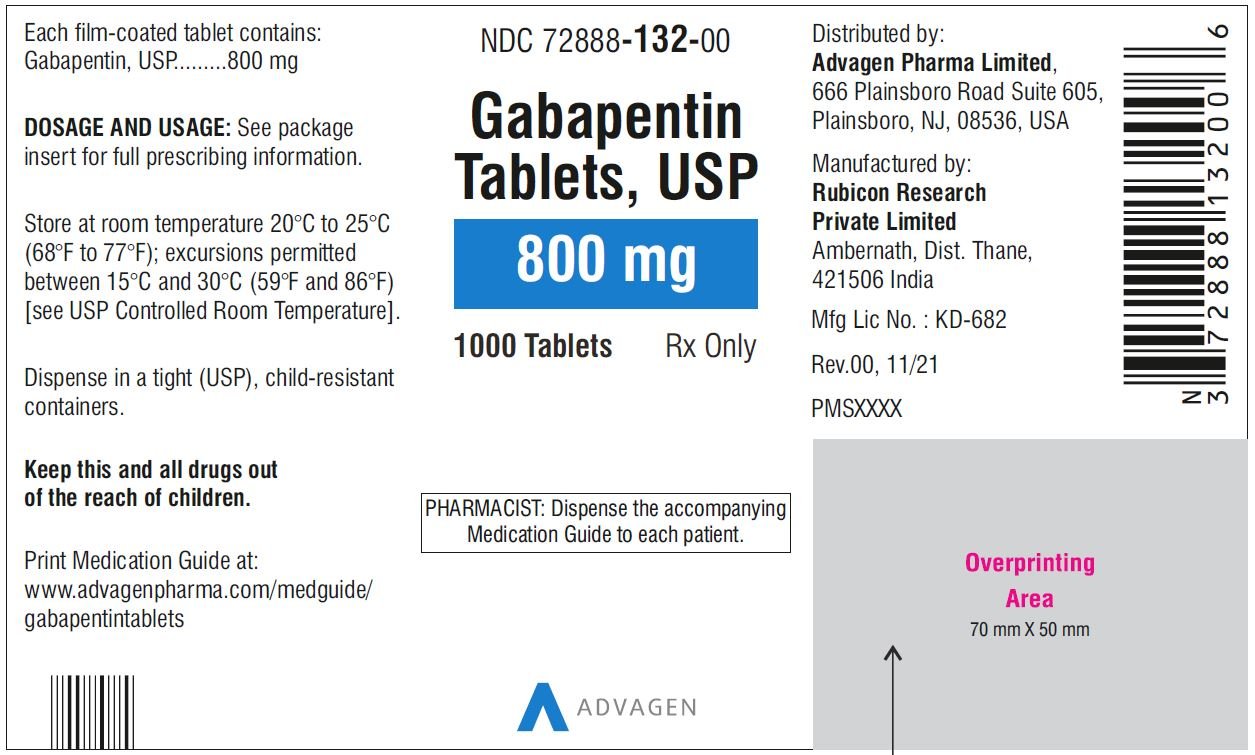
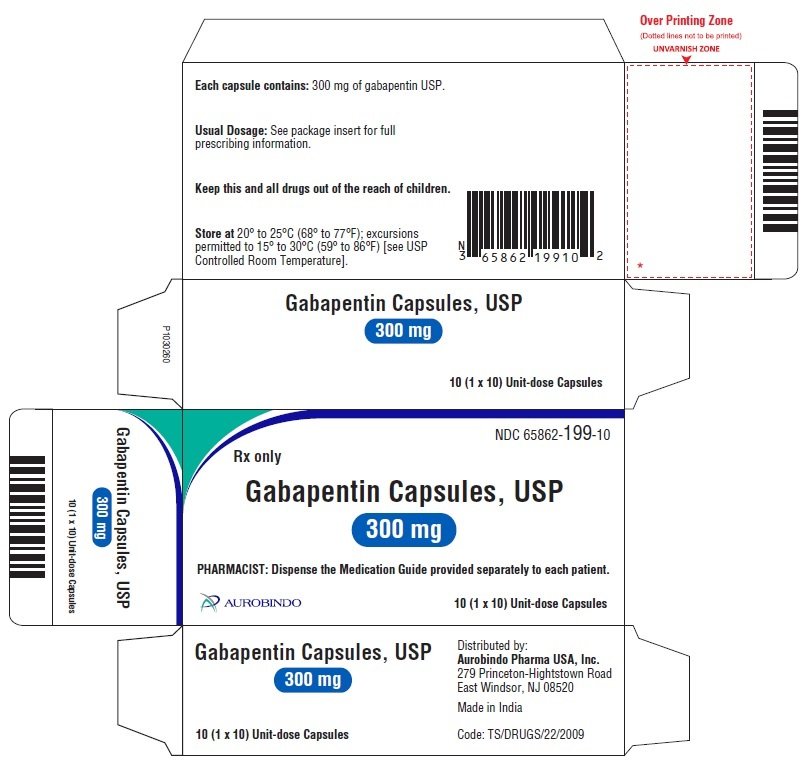
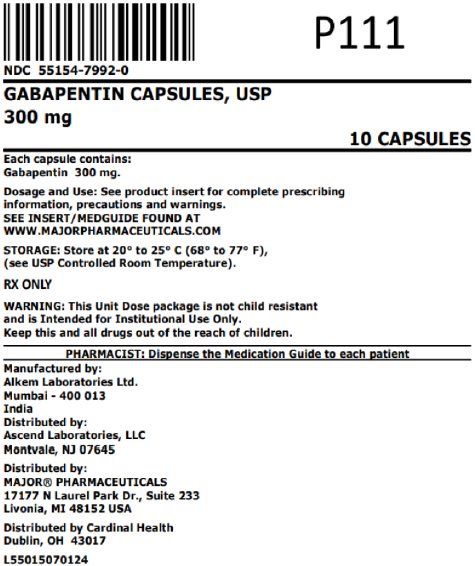
(gabapentin enacarbil) Extended-Release Tablets are indicated for the treatment of moderate-to-severe primary Restless Legs Syndrome (RLS) in adults. HORIZANT is not recommended for patients HORIZANT ® (gabapentin enacarbil) Extended-Release Tablets are indicated for the treatment of moderate-to-severe primary Restless Legs Syndrome (RLS) in adults. HORIZANT is not recommended for patients who are required to sleep during the daytime and remain awake at night. Driving performance studies conducted with a prodrug of gabapentin (gabapentin enacarbil tablet, extended-release) indicate that gabapentin may cause significant driving impairment. Prescribers and patients should be aware that patients' ability to assess their own driving competence, as well as their ability to assess the degree of somnolence Released gabapentin is not appreciably metabolized in humans. Neither gabapentin enacarbil nor gabapentin are substrates, inhibitors, or inducers of the major cytochrome P450 enzymes (CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4). Gabapentin enacarbil is neither a substrate nor an inhibitor of P-glycoprotein in vitro. (gabapentin enacarbil) Extended-Release Tablets Read this Medication Guide before you start taking HORIZANT and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment. HORIZANT® (gabapentin enacarbil) Extended-Release Tablets are indicated for the treatment of moderate-to-severe primary Restless Legs Syndrome (RLS) in adults. HORIZANT is not recommended for patients who are required to sleep during the daytime and remain awake at night. References: 1. Horizant [package insert] Woburn, MA: Azurity Pharmaceuticals, Inc.; 2022. 2. Cundy KC, Sastry S, Luo W, et al. Clinical pharmacokinetics of XP13512, a Gabapentin may be administered as the oral capsule. Dosages up to 50 mg/kg/day have been administered in a long-term clinical study. The maximum time interval between doses should not exceed 12 hours. TABLE 1. Gabapentin Dosage Based on Renal Function. Horizant package insert / prescribing information for healthcare professionals. Gabapentin enacarbil is a white to off-white crystalline solid with a melting HORIZANT® (gabapentin enacarbil) Extended-Release Tablets are indicated for the treatment of moderate-to-severe primary Restless Legs Syndrome (RLS) in adults. HORIZANT is not recommended for patients who are required to sleep during the daytime and remain awake at night. Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly *The recommended dosage of Horizant® for RLS is 600 mg taken once daily with food at about 5 PM. A daily dose of 1200 mg provided no additional benefit compared with the 600 mg dose and caused an increase in adverse reactions. gabapentin relative to other gabapentin products [see Clinical Pharmacology (12.3)]. The safety and effectiveness of HORIZANT in patients with epilepsy have not been studied. 5.4 Suicidal Behavior and Ideation HORIZANT (gabapentin enacarbil) is a prodrug of gabapentin, an antiepileptic drug (AED). AEDs Gabapentin may be administered as the oral capsule. Dosages up to 50 mg/kg/day have been administered in a long-term clinical study. The maximum time interval between doses should not exceed 12 hours. HORIZANT® (gabapentin enacarbil) Extended-Release Tablets are indicated for the treatment of moderate-to-severe primary Restless Legs Syndrome (RLS) in adults. HORIZANT is not recommended for References: 1. Horizant [package insert] Woburn, MA: Azurity Pharmaceuticals, Inc.; 2022. 2. Cundy KC, Sastry S, Luo W, et al. Clinical pharmacokinetics of XP13512, a HORIZANT (gabapentin enacarbil) Extended-Release Tablets are indicated for the management of postherpetic neuralgia (PHN) in adults. 2 DOSAGE AND ADMINISTRATION . Tablets should be swallowed Gabapentin enacarbil: Commercially available as extended-release tablets (Horizant). Because of differences in pharmacokinetics that affect frequency of administration, gabapentin enacarbil extended-release tablets are not interchangeable with other gabapentin preparations. (See Pharmacokinetics.) HORIZANT® (gabapentin enacarbil) Extended-Release 1.1 Treatment of Restless Legs Syndrome - HORIZANT® is indicated for the treatment of moderate-to-severe primary Restless Legs Syndrome (RLS) in adults. HORIZANT is not recommended for patients Tablets should be swallowed whole and should not be cut, crushed, or chewed. References: 1. Horizant [package insert] Woburn, MA: Azurity Pharmaceuticals, Inc.; 2022. 2. Cundy KC, Sastry S, Luo W, et al. Clinical pharmacokinetics of XP13512, a
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |