Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
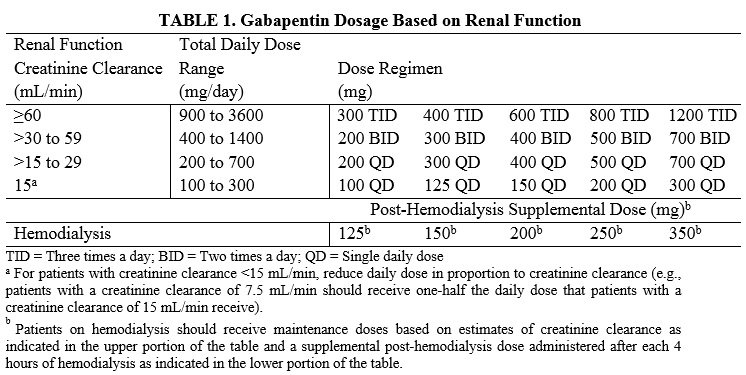 |  |
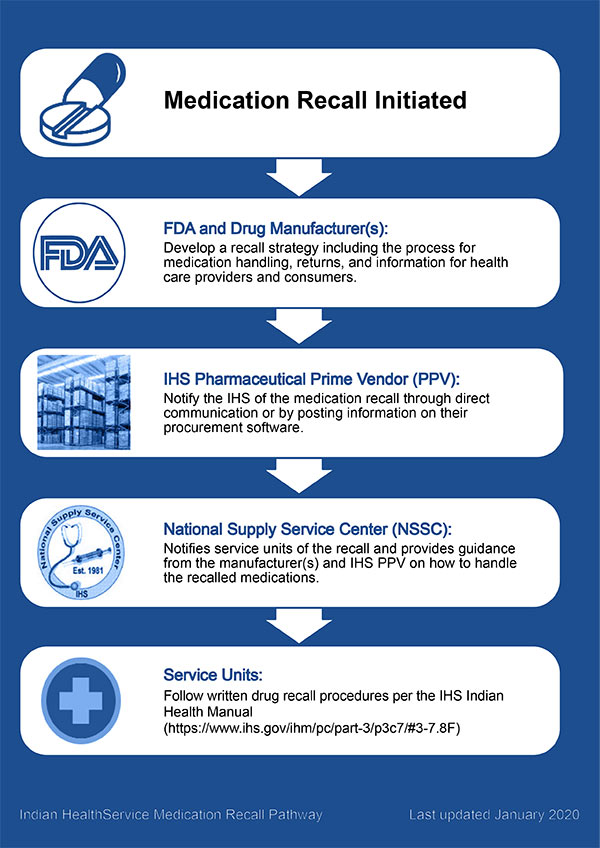
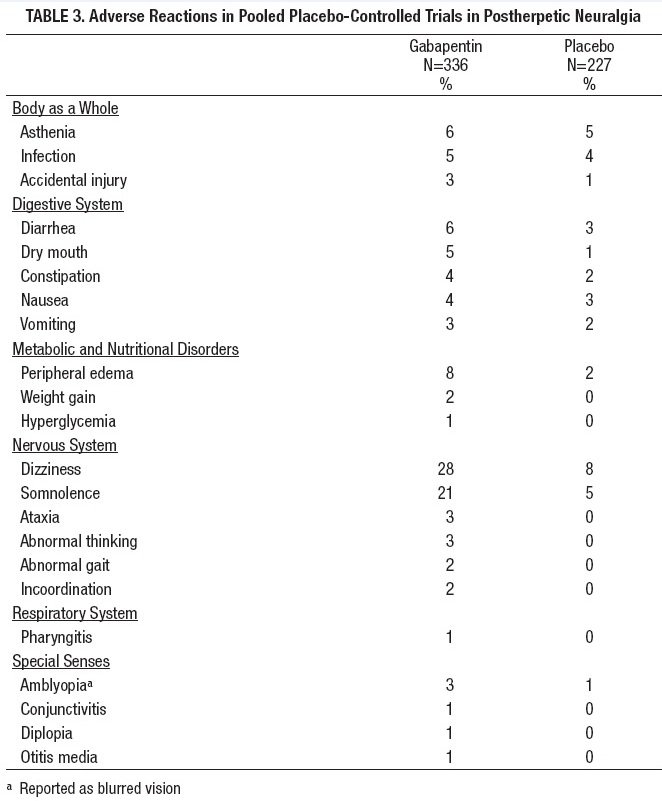
FDA Recalls Class III Description: Gabapentin Tablets, USP 600 mg, 500 tablets per bottle, RX Only, Manufactured by ScieGen Pharmaceuticals Inc., Hauppauge, NY 11788, NDC: 50228-177-05. Reports of empty capsules prompt a nationwide voluntary recall of one lot of 300-mg capsules of the antiepileptic drug gabapentin. The Drug Recall Report is for prescription drugs that are have recently been issued recalls by the U.S. Food and Drug Administration (FDA). Gabapentin 100mg The last Recall Enforcement Report for Gabapentin with NDC 76282-406 was initiated on 05-14-2020 as a Class III recall due to failed impurities/degradation specifications; failure of impurity a test at the 12-month long-term stability testing. Visit Recalls, Market Withdrawals, & Safety Alerts for all FDA-regulated products. Follow FDA Recall Information on X (formerly Twitter). FDA provides a searchable list of recalled products. FDA Recalls Class III Description: Gabapentin Tablets, USP 600 mg, packaged in Cartons of 100 tablets (10 tablets per blister pack x 10), Rx Only, Distributed by: Aurobindo Pharma USA, Inc. East Windsor, NJ 08520 Distributed by: Major Pharmaceuticals 17177 N Laurel Park Dr., Suite 233 Livonia, MI 48152 USA, NDC 0904-6823-61 Aurobindo Pharma USA, Inc, of Dayton, NJ, has issued a voluntary recall of one lot (Lot Number GESB14011-A) of gabapentin capsules, USP 300 mg, 100-count bottles to the consumer level after finding that some capsules were empty. The Harvard Drug Group is pulling 3984 cartons of gabapentin tablets after a foreign tablet was found in a carton, according to the May 17, 2023, US Food and Drug Administration (FDA) Enforcement Report. Gabapentin has been pitched for so many different conditions that a drug company executive infamously called it “snake oil.” Gabapentin is FDA-approved for epilepsy and neuropathic pain caused by shingles, but is often prescribed off-label for depression, ADHD, migraine, fibromyalgia, bipolar disorder and postoperative pain. FDA provides a searchable list of recalled products. Drug recalls are actions taken by a firm to remove a product from the market and may be conducted on a firm's own initiative, by FDA request FDA is warning that serious, life-threatening, and fatal respiratory depression has been reported with the gabapentinoids, gabapentin (Neurontin, Gralise, Horizant) and pregabalin (Lyrica, Lyrica The Harvard Drug Group is pulling 3984 cartons of gabapentin tablets after a foreign tablet was found in a carton, according to the May 17, 2023, US Food and Drug Administration (FDA) Enforcement Report. The recall affects gabapentin tablets, 600 mg, packaged in 100-tablet cartons (10 blister packs containing 10 tablets each, NDC 0904-6823-61 The U.S. Food and Drug Administration (FDA) has reported that Aurobindo Pharma USA is voluntarily recalling Gabapentin 300 mg capsules from lot GESB14011-A in 100-count bottles. The product lot affected was found to include some empty capsules; thus, if taken by a person with seizures, they would not get any active seizure medication. FDA is requiring new warnings about the risk of serious breathing difficulties that can lead to death in patients who use gabapentanoids with opioid pain medicines or other drugs that depress the Drug Recall Enforcement Report Class II voluntary initiated by Granules Pharmaceuticals Inc., originally initiated on 07-31-2024 for the product Gabapentin Tablets, USP, 600 mg, 500-count bottles, Rx only, Manufactured by: Granules India Limited Hyderabad-500 081, India, Manufactured for: Granules Pharmaceuticals Inc., Chantilly, VA NDC 70010-227-05 The product was recalled due to presence of Get an alert when a recall is issued. Do not use • if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. The U.S. Food and Drug Administration (FDA) has reported that Aurobindo Pharma USA is voluntarily recalling Gabapentin 300 mg capsules from lot GESB14011-A in 100-count bottles. The product lot affected was found to include some empty capsules; thus, if taken by a person with seizures, they would not get any active seizure medication. The drug product(s) included in any of the potential signals of serious risks/new safety information listed below may also include the generic version of the drug product(s), if there are generic The last Recall Enforcement Report for Gabapentin with NDC 0904-6823 was initiated on 04-24-2023 as a Class III recall due to product mixup: one foreign tablet found in product. The latest recall number for this product is D-0570-2023 and the recall is currently terminated as of 04-30-2024 .
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |