Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
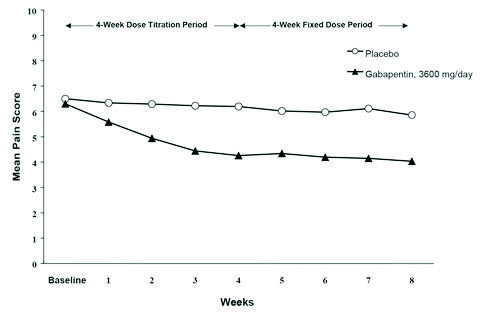
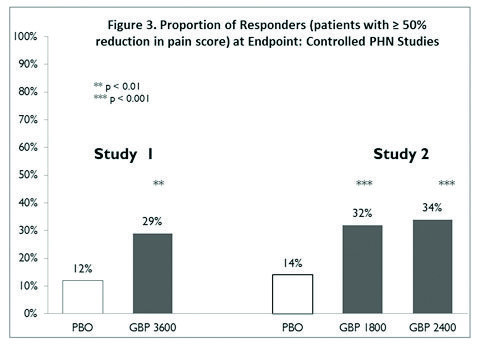
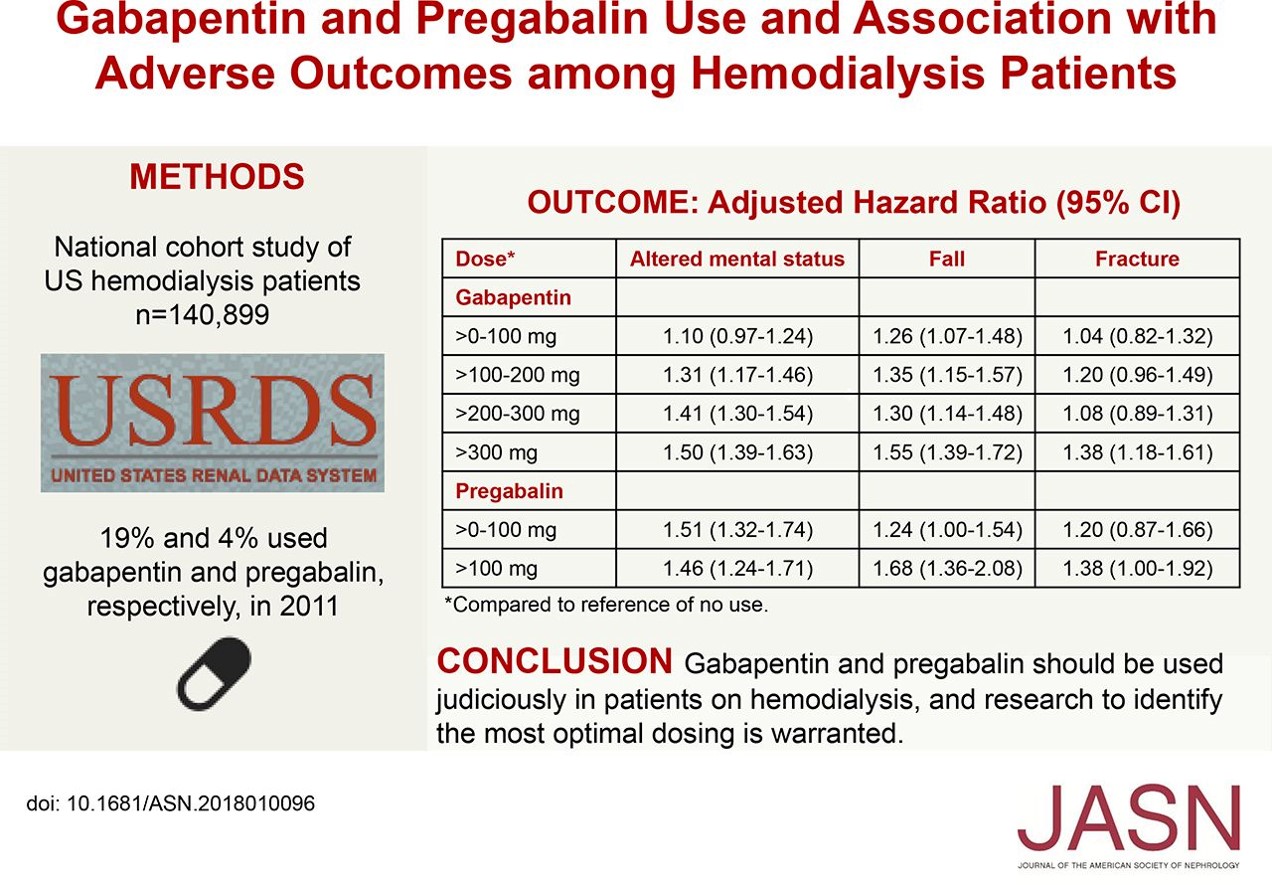
Gabapentin Oral Solution is a prescription medicine used to treat: z Pain from damaged nerves (postherpetic pain) that follows healing of shingles (a painful rash that comes after a herpes zoster infection) in adults. NEURONTIN safely and effectively. See full prescribing information for NEURONTIN. NEURONTIN ® (gabapentin) capsules, for oral use NEURONTIN ® (gabapentin) tablets, for oral use NEURONTIN ® (gabapentin) oral solution Initial U.S. Approval: 1993 ----- Warnings and Pr ecautions, Respiratory Depression (5.7) 04/2020 Gabapentin oral solution may cause serious or life-threatening allergic reactions that may affect For more information go to www.amneal.com. or call 1-877-835-5472. Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of SOLUTION safely and effectively. See full prescribing information for GABAPENTIN ORAL SOLUTION. GABAPENTIN oral solution Initial U.S. Approval: 1993 RECENT MAJOR CHANGES Warnings and Precautions, Respiratory Depression ( 5.7) 04/2020 INDICATIONS AND USAGE Gabapentin oral solution is indicated for: Postherpetic neuralgia in adults ( 1) The recommended maintenance dose of gabapentin oral solution in patients 5 to 11 years of age is 25 mg/kg/day to 35 mg/kg/day, given in three divided doses. Gabapentin oral solution may be administered as the oral solution, capsule, or tablet, or using combinations of these formulations. Gabapentin capsules are indicated for: In adults with postherpetic neuralgia, gabapentin capsules may be initiated on Day 1 as a single 300 mg dose, on Day 2 as 600 mg/day (300 mg two times a day), and on Day 3 as 900 mg/day (300 mg three times a day). Gabapentin Oral Solution is indicated for: Postherpetic neuralgia in adults ( 1) Adjunctive therapy in the treatment of partial onset seizures, with and without secondary generalization, in adults and pediatric patients 3 years and older with epilepsy ( 1) Order Gabapentin 250 mg / 5 mL Solution 473 mL (16 oz.) by Amneal 65162069890. Call Us. Customer Service 855.571.2100. Need help with SupplyManager? 800.422.0280. Accounts Receivable Gabapentin oral solution is indicated for: Postherpetic neuralgia in adults (1) Adjunctive therapy in the treatment of partial onset seizures, with and without secondary generalization, in adults and pediatric patients 3 years and older with epilepsy (1) Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and SOLUTION safely and effectively. See full prescribing information for GABAPENTIN ORAL SOLUTION. GABAPENTIN oral solution Initial U.S. Approval: 1993 RECENT MAJOR CHANGES Warnings and Precautions, Respiratory Depression ( 5.7) 04/2020 INDICATIONS AND USAGE Gabapentin Oral Solution is indicated for: Postherpetic neuralgia in adults ( 1) Gabapentin Oral Solution package insert / prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions and pharmacology. Gabapentin oral solution is indicated for: Management of postherpetic neuralgia in adults - Adjunctive therapy in the treatment of partial onset seizures, with and without secondary Gabapentin oral solution is indicated for: Management of postherpetic neuralgia in adults Adjunctive therapy in the treatment of partial onset seizures, with and without We are also excited about our work to bring patients more affordable biologic therapy options through our Biosciences, which includes a broad portfolio of institutional injectables as well as Amneal’s growing biosimilars portfolio. And we’re expanding our business across select international markets. Learn more about our product portfolios: The NDC Packaged Code 65162-698-90 is assigned to a package of 473 ml in 1 bottle of Gabapentin, a human prescription drug labeled by Amneal Pharmaceuticals Llc. The product's dosage form is solution and is administered via oral form. The NDC code 65162-698 is assigned by the FDA to the product Gabapentin which is a human prescription drug product labeled by Amneal Pharmaceuticals Llc. The product's dosage form is solution and is administered via oral form. 3 days. The recommended maintenance dose of NEURONTIN in patients 3 to 4 years of age is 40 mg/kg/day, given in three divided doses. The recommended maintenance dose of NEURONTIN in patients 5 to 11 years of age is 25 mg/kg/day to 35 mg/kg/day, given in three divided doses. NEURONTIN may be administered as the oral solution, capsule, or tablet, or GABAPENTIN- gabapentin suspension Greenstone LLC-----HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use GABAPENTIN safely and effectively. See full prescribing information for GABAPENTIN. Gabapentin capsules, for oral use Gabapentin tablets, for oral use Gabapentin oral solution
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |