Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
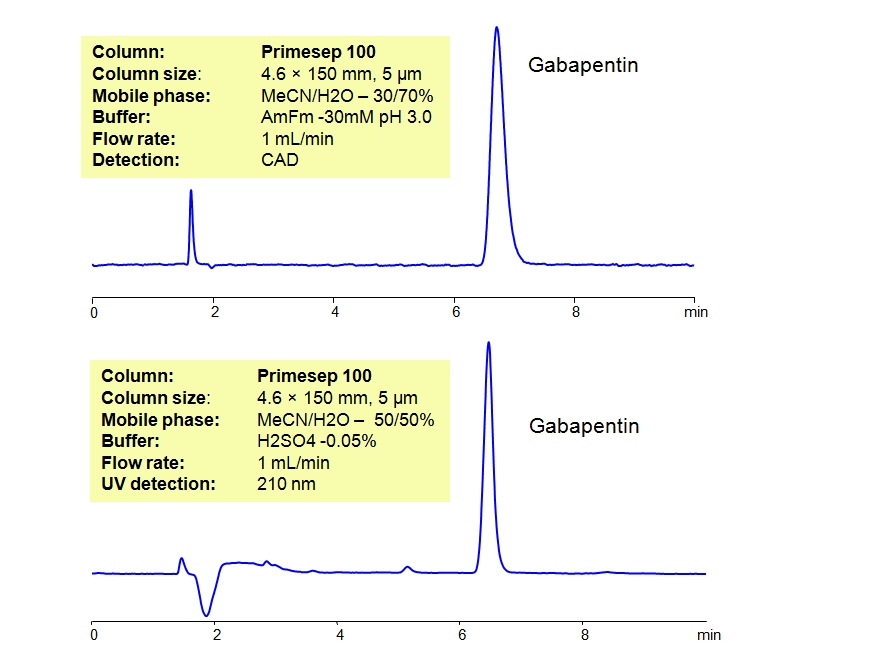
Confirmation Method: Gabapentin Page . 1. of . 8. Approved by the State Toxicologist Effective date: 6/15/20 Printed Copies are Uncontrolled TCg12735 – Revision: 5 . CONFIRMATION OF GABAPENTIN BY LIQUID CHROMATOGRAPHY- MASS SPECTROMETRY . 35.1 METHOD . This test method may be used to confirm the presence of gabapentin (GABA) in biological A rapid, sensitive and accurate high performance liquid chromatography with UV detection method was developed and validated for the quantification of gabapentin in bulk, pharmaceutical formulation and human urine samples. A USP stability-indicating assay method was adapted and optimized for gabapentin analysis. 15 Samples were analyzed with the use of Varian-920 high-performance liquid chromatography with a quaternary gradient pump, autosampler (50 μL sample loop), a UV-Vis detector, and Galaxie chromatographic software. The stationary phase consisted of a Bahrami G, Mohammadi B. Sensitive microanalysis of gabapentin by high-performance liquid chromatography in human serum using pre-column derivatization with 4-chloro-7-nitrobenzofurazan: Application to a bioequivalence study. J. Chromatogr B. 2006;837:24–28. doi: 10.1016/j.jchromb.2006.03.056. [Google Scholar] 4. A simple, sensitive and rapid liquid chromatography/tandem mass spectrometry (LC–MS/MS) method was developed and validated for the quantification of gabapentin, a new antiepileptic drug, in human plasma using its structural analogue, 1,1-cyclohexane diacetic acid monoamide (CAM) as internal standard. Sensitive microanalysis of gabapentin by high-performance liquid chromatography in human serum using pre-column derivatization with 4-chloro-7-nitrobenzofurazan: Application to a bioequivalence study Author links open overlay panel Gholamreza Bahrami a b , Bahareh Mohammadi a A method of liquid chromatography/mass spectrometry has been developed and validated to measure gabapentin and the results were monitored for one year; a sample of protein's precipitation has been prepared with acetonitrile having internal isotopically standards with label, using reverse phase liquid chromatography ultraperformance separation A rapid, sensitive and specific analytical method was developed and validated to quantify gabapentin in human plasma using acetaminophen as an internal standard. The method employs a single plasma protein precipitation. A simple, sensitive and rapid liquid chromatography/tandem mass spectrometry (LC-MS/MS) method was developed and validated for the quantification of gabapentin, a new antiepileptic drug, in human A rapid and simple method for determination of the novel antiepileptic compound gabapentin [1-(aminomethyl)cyclohexaneacetic acid] in plasma is described. Blank human plasma was spiked with gabapentin (1.0–10.0 μg/ml) and internal standard [1-(aminomethyl)-cycloheptaneacetic acid; 5.0 μg/ml]. Abstract. An accurate, highly sensitive, and precise method for quantitative analysis of tramadol (TMD) and gabapentin (GBP) by high performance liquid chromatography and tandem mass spectrometry in human plasma was proposed and validated successfully using venlafaxine and pregabalin as internal standards (ISTDs), respectively. A sensitive high-performance liquid chromatography (HPLC) method using UV detection for the determination of gabapentin in human plasma has been developed. In this method, gabapentin was extracted from human plasma with a reversed-phase solid-phase extraction (SPE) cartridge followed by derivatization with phenylisothiocyanate. Gabapentin was quantitated in feline plasma by liquid chromatography–mass spectrometry analysis of extracted plasma samples, according to a modification of the methods reported elsewhere. 11 – 13 The calibration standards were prepared as follows. Stock solutions were made by dissolving 10.0 mg gabapentin standard in 10.0 mL acetonitrile (ACN). An analytical method for the determination of gabapentin in serum obtained from venous blood samples has been developed using high-performance liquid chromatography (HPLC)–tandem mass spectrometry. In addition, a comparative study between capillary plasma samples and venous serum samples was carried out. Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) Method to Quantify Gabapentin and Pregabalin in Urine Methods Mol Biol . 2019:1872:119-127. doi: 10.1007/978-1-4939-8823-5_12. This test method may be used to confirm the presence of gabapentin (GABA) in biological samples. Quantitative results obtained through the use of this method will A simple HPLC method was developed and validated for quantitation of gabapentin in pure form. The HPLC separation was achieved on a C18 5 μm Waters column (150 mm × 4.6 mm) using a mobile phase of methanol - potassium dihydrogen orthophosphate A rapid, sensitive and selective method for the determination of gabapentin in human plasma was developed using hydrophilic interaction liquid chromatography/tandem mass spectrometry (HILIC/MS/MS). A rapid and simple method for determination of the novel antiepileptic compound gabapentin [1-(aminomethyl)cyclohexaneacetic acid] in plasma is described. Blank human plasma was spiked with gabapentin (1.0-10.0 micrograms/ml) and internal standard [1-(aminomethyl)-cycloheptaneacetic acid; 5.0 microg A sensitive validated liquid chromatography-tandem mass spectrometric method (LC-MS/MS) for gabapentin (GB) in human plasma has been developed and applied to pharmacokinetic (PK) and
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |