Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
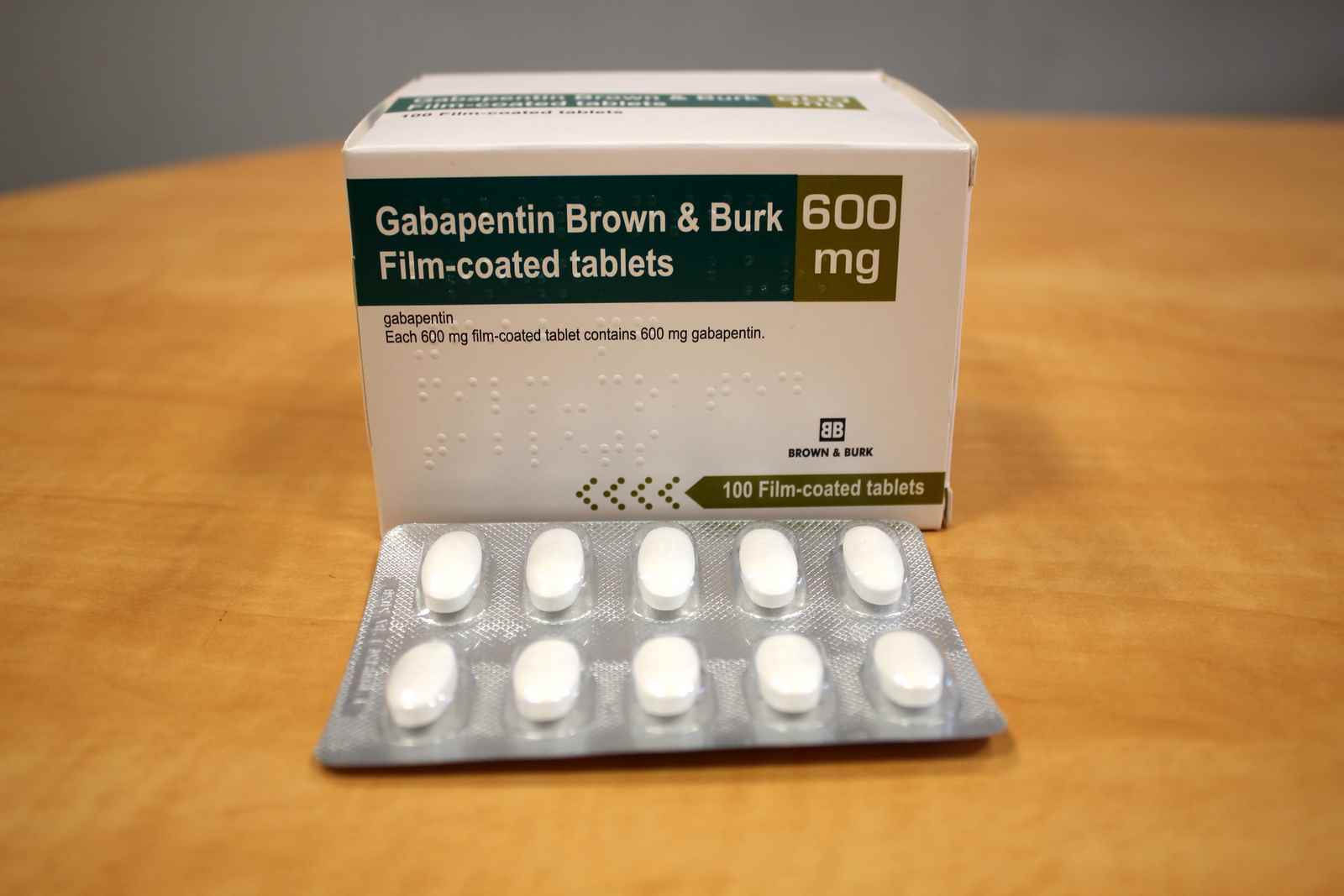 |  |
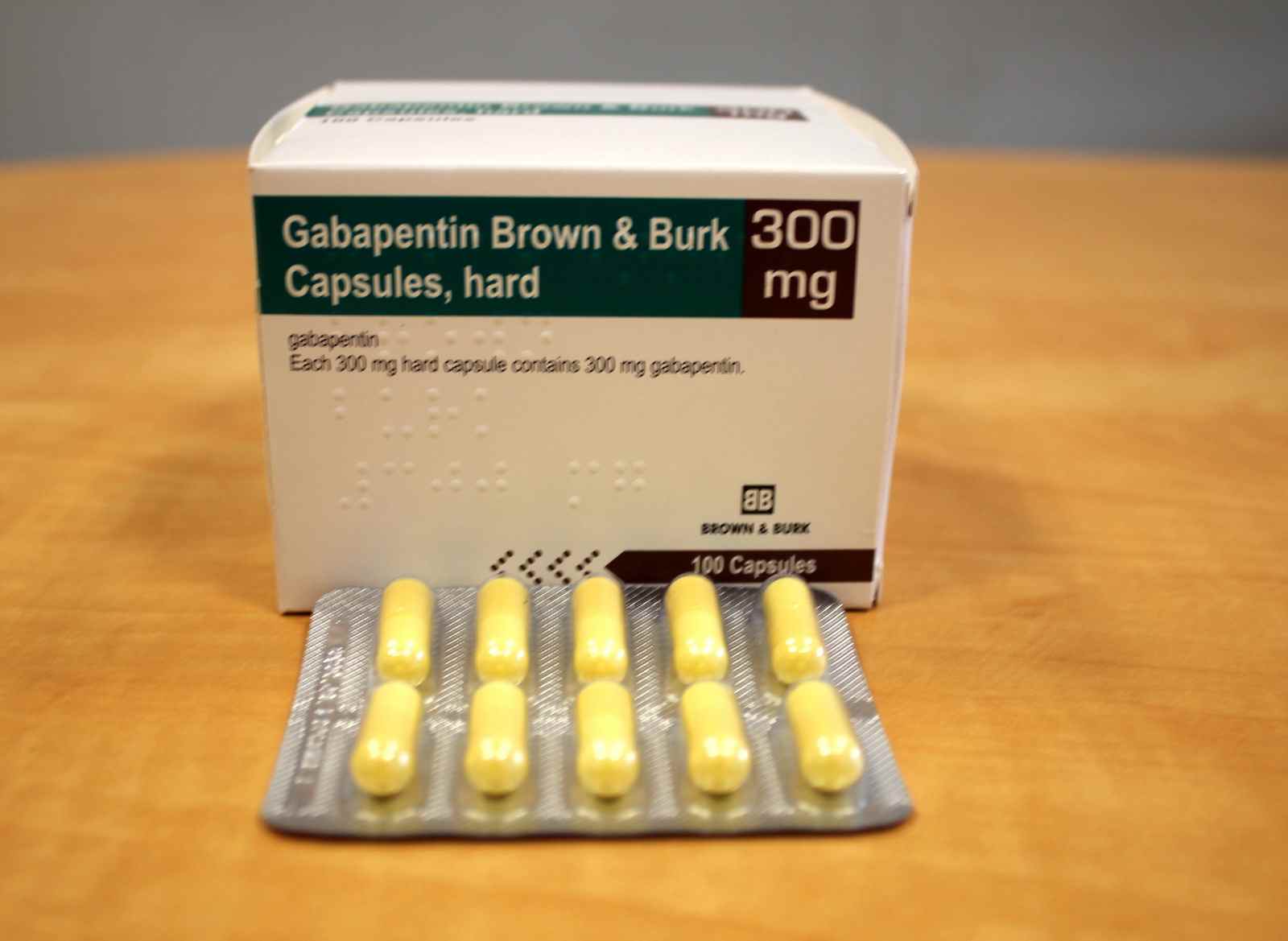
Updates: Go to lucodes.ca/lu while online for the most up to date version. Ontario Limited Use Code lookup with your browser for all platforms. You can save the page for offline use as well. drug profile on the ODB e-Formulary for the details of the Limited Use (LU) code and criteria, and/or any associated Therapeutic Notes (TN). List of products in the National Drug Code with proprietary name gabapentin. Gabapentin is used with other medications to prevent and control seizures. It is also used to relieve nerve pain following shingles (a painful rash due to herpes zoster infection) in adults. Limited Use (LU) Code - Ontario. The following topics are applicable to ON pharmacies only. Limited Use (LU) codes, sometimes referred to as Medical Reason (MR) codes, are required by DB and TR for a patient to receive coverage for certain medications. The codes must be provided by the prescriber. 02477912 AG -Gabapentin 100mg Cap ANG 0.0416 02477920 AG-Gabapentin 300mg Cap ANG 0.1012 02477939 AG-Gabapentin 400mg Cap ANG 0.1206 (Interchangeable with Neurontin – GB) DIN/PIN Product Name Strength Dosage Form Mfr DBP . 02481588 AG -Pantoprazole Sodium 40mg DR Tab ANG 0.2016 (Interchangeable with Pantoloc – LU) Note: Less costly anticonvulsant therapies may include the following: Phenytoin, Carbamazepine, Gabapentin, Lamotrigine, Vigabatrin, Topiramate, etc. LU Authorization Period: Indefinite Revised Limited Use (LU) Criteria. Applies to various strengths of Aptiom (eslicarbazepine acetate), Fycompa (perampanel) and Vimpat (lacosamide). Please see the SOC document for details. Revised LU Code 430 Reason For Use Code Clinical Criteria; 441: In combination with low-dose ASA for patients with: - Non-ST elevation acute coronary syndrome (ACS)* (unstable angina or myocardial infarction [MI]); OR - ST-segment elevation myocardial infarction (STEMI); OR - Stent thrombosis while taking clopidogrel plus low-dose ASA. Drug(s) LU Clinical criteria code Cipro (ciprofloxacin) 332 333 334 For the treatment of patients with: SST/BJ (Gram negative bacteria): Skin/soft tissue and bone/joint infection due to gram negative bacteria; severe diabetic foot infection; severe otitis externa; decubitus ulcers. GU Tract: Urinary tract infection/prostatitis/epididymitis Authorization Period: Indefinite! ® Neurontin 100mg;300mg;400mg Cap;GABAPENTIN136 [Change from LU to General Benefit on Oct 28, 2010] As adjunctive therapy in the treatment of seizure disorders where control by other listed anticonvulsants has been unsatisfactory. The Limited Use (LU) Codes 124, 125, 126 and 225 and clinical criteria are the same as for the currently listed Creon 25 product DIN 01985205. Generic Name: ADALIMUMAB . DIN/PIN Brand Name Strength Dosage Form Mfr DBP . 02502682 Idacio 40mg/0.8mL Inj Sol-0.8mL Pref Syr (Preservative-Free) FKC 471.2700/ Pref Syr 00360279 Chlorthalidone 50mg Tab AAP 0.1419 02393239 Apo-Lamivudine HBV 100mg Tab APX 2.6154 02244727 Apo-Medroxy 5mg Tab APX 0.2365 Alternative in liver disease = Acamprosate 333mg po TID, LU code 531. Gabapentin 400mg PO TID x 7 days. $0.13 per pill. For use as an adjunct after benzodiazepine load in ED. Contraindications: – Liver failure – Do not use as monotherapy if high risk for severe withdrawal/seizure/delirium The Limited Use (LU) codes 600-607, 609 and 611 and clinical criteria are the same as for the currently listed Hadlima 40mg/0.8mL products (DIN 02473097 and 02473100). ATC Code. N02BF01 Interchangeable Products Apo-Gabapentin: 1.7393: NA: 02432080: Gabapentin: 1.7393: NA LU Clinical Criteria NO EAP Criteria 666 mg PO three times daily x 14 days (333 mg if CrCl 30–50 ml/min), LU code 531 Contraindications: Severe renal impairment (CrCl < 30), known allergy, nursing Usual side e˜ect: Diarrhea (can start/stay on lower dose, but settles over time) GABAPENTIN (o˜-label use) Relieves mild and/or ongoing withdrawal, promotes abstinence. Limited Use Note(s) Reason For Use Code Clinical Criteria; 315: For the treatment of depression. LU Authorization Period: Indefinite Reason For Use Code Clinical Criteria; 430: As adjunctive therapy in the treatment of patients with partial onset seizures who have had an inadequate response or have significant intolerance to at least 3 less costly anticonvulsant therapies; AND Patients are under the care of a physician experienced in the treatment of epilepsy. drug profile on the ODB e-Formulary for the details of the Limited Use (LU) code and criteria, and/or any associated Therapeutic Notes (TN). DIN/PIN
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |