Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
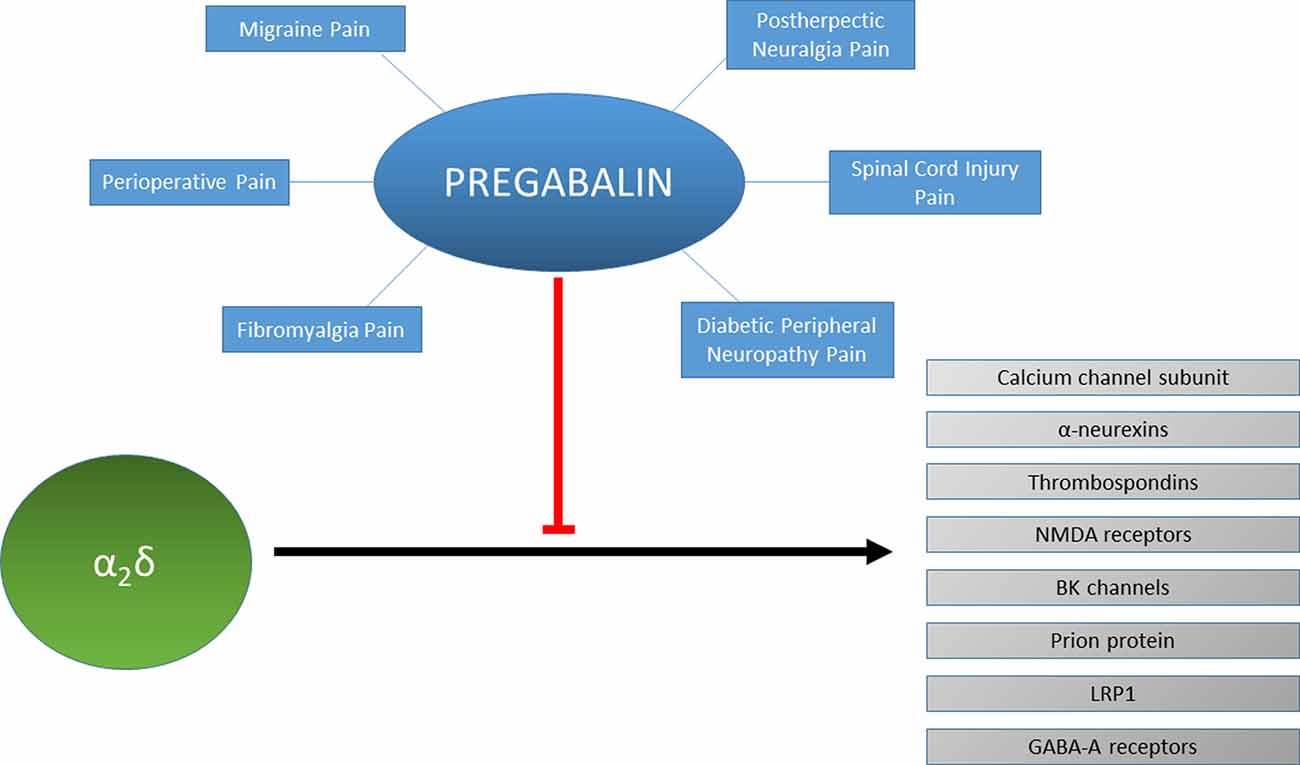
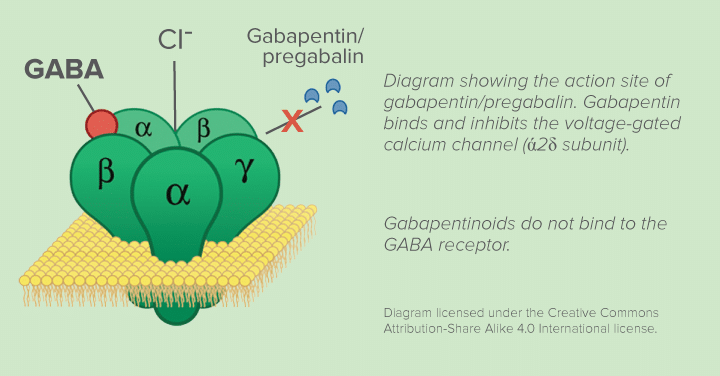
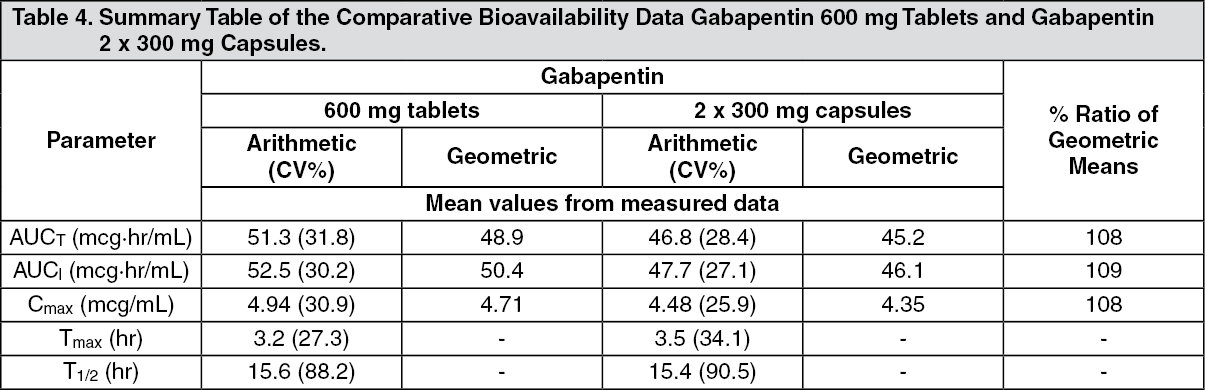
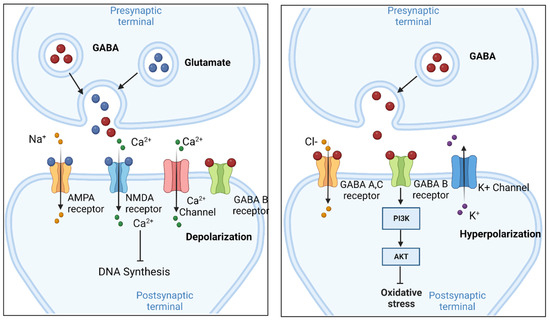
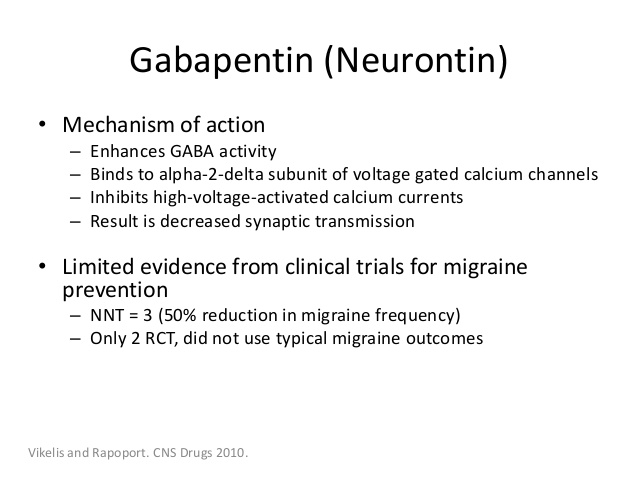
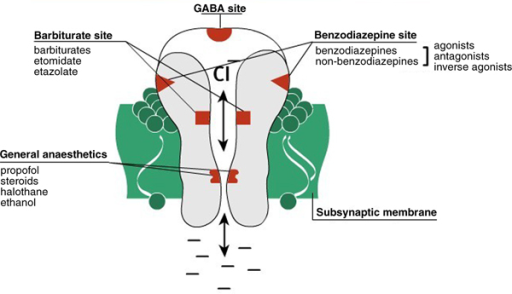
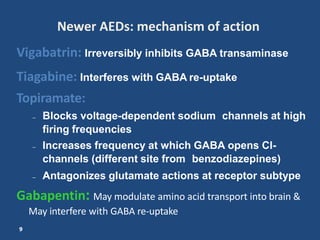
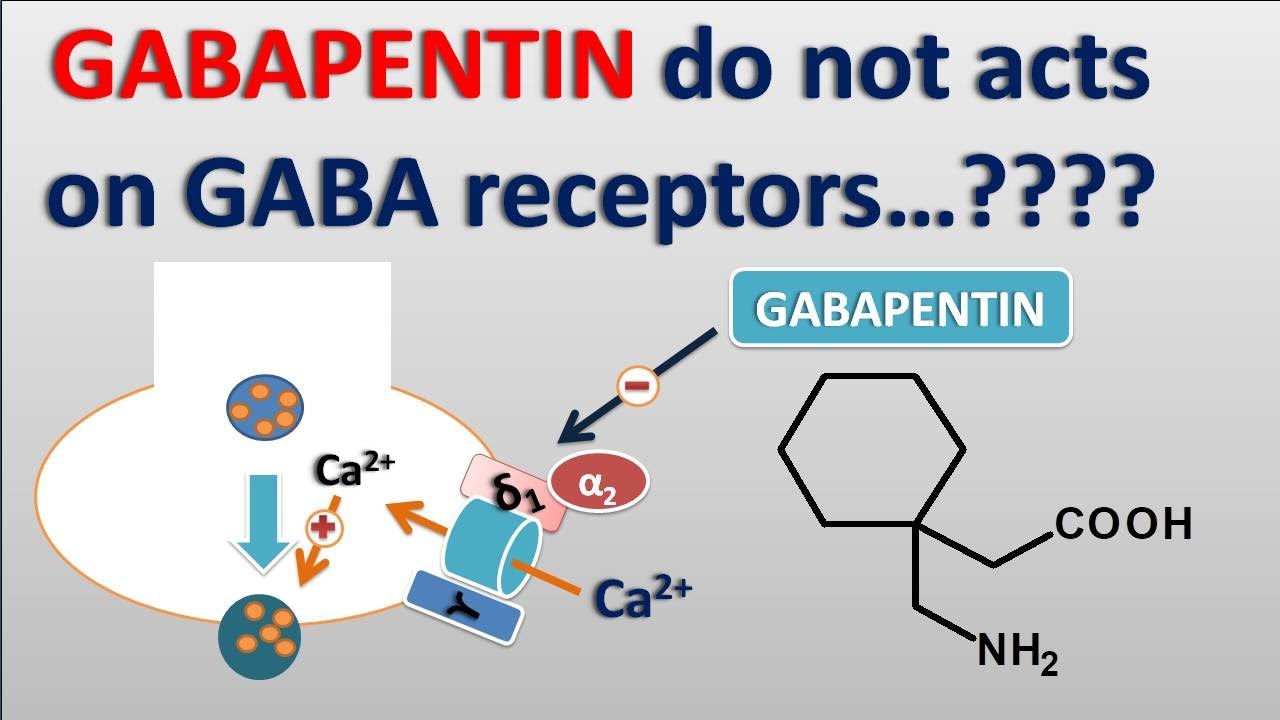
Mechanism of Action. Gabapentin is structurally related to GABA. However, it does not bind to GABA A or GABA B receptors, and it does not appear to influence synthesis or uptake of GABA. High affinity gabapentin binding sites have been located throughout the brain; these sites correspond to the presence of voltage-gated calcium channels Originally designed as analogs of GABA, the gabapentinoids bind to the α 2 δ ‐1 and α 2 δ ‐2 auxiliary subunits of calcium channels, though only the former has been implicated in the development of neuropathy in animal models. Although the exact mechanism of action with the GABA receptors is unknown, researchers know that gabapentin freely passes the blood-brain barrier and acts on neurotransmitters. Gabapentin has a cyclohexyl group to the structure of the neurotransmitter GABA as a chemical structure. Mechanism of action. Gabapentin has no direct GABAergic action and does not block GABA uptake or metabolism. Gabapentin blocks the tonic phase of nociception induced by formalin and carrageenan, and exerts a potent inhibitory effect in neuropathic pain models of mechanical hyperalgesia and mechanical/thermal allodynia. Gabapentin is an anti-epileptic drug but its use has expanded to treat multiple other diseases including post-herpetic neuralgia, neuropathic pain, and spasticity. The mechanism of action is not fully understood but may be related to gabapentin’s action on calcium channels leading to diminution of excitatory neurotransmitters. Gabapentin is a potent activator of voltage-gated potassium channels KCNQ3 and KCNQ5, even at low nanomolar concentrations. However, this activation is unlikely to be the dominant mechanism of gabapentin's therapeutic effects. [90] Although the cellular mechanisms of pharmacological actions of gabapentin (Neurontin ®) remain incompletely described, several hypotheses have been proposed. It is possible that different mechanisms account for anticonvulsant, antinociceptive, anxiolytic and neuroprotective activity in animal models. Mechanism of action: By inhibiting the voltage-gated calcium channels in the CNS, gabapentin reduces the release of excitatory neurotransmitters (mostly noradrenaline, dopamine and serotonin), and therefore decreases epileptogenesis. Clinical effects Mechanism of action. Gabapentin has no direct GABAergic action and does not block GABA uptake or metabolism. Gabapentin blocks the tonic phase of nociception induced by formalin and carrageenan, and exerts a potent inhibitory effect in neuropathic pain models of mechanical hyperalgesia and mechanical/thermal allodynia. Gabapentin (GBP) was originally developed as a potential agonist for Gamma-Amino-Butyric-Acid (GABA) receptors, aiming to inhibit the activation of pain-signaling neurons. Contrary to initial expectations, it does not bind to GABA receptors. Instead, it exhibits several distinct pharmacological activities, including: (1) binding to the alpha-2-delta protein subunit of voltage-gated calcium Mechanism of action. The precise mechanism through which gabapentin exerts its therapeutic effects is unclear. 16,17 The primary mode of action appears to be at the auxillary α2δ-1 subunit of voltage-gated calcium channels (though a low affinity for the α2δ-2 subunit has also been reported). 10,8,14 The major function of these subunits is Gabapentin and pregabalin are structurally related compounds with recognized efficacy in the treatment of both epilepsy and neuropathic pain. The pharmacological mechanisms by which these agents exert their clinical effects have, until recently, remained unclear. The interaction of gabapentin and pr Gabapentin prevents pain responses in several animal models of hyperalgesia and prevents neuronal death in vitro and in vivo with models of the neurodegenerative disease amyotrophic lateral sclerosis (ALS). Gabapentin is also active in models that detect anxiolytic activity. One of the primary mechanisms of gabapentin involves its interaction with voltage-gated calcium channels (VGCCs). Specifically, gabapentin binds to the alpha-2-delta subunit of these channels. VGCCs play a crucial role in the release of neurotransmitters by regulating calcium influx into neurons. As with many other agents, GBP was licensed for the treatment of epilepsy with little or no understanding of its mechanism of action. Continued research and the parallel development of PGB have contributed to a contemporary pharmacological view of GBP (and PGB) as drugs with multiple modest cellular effects at therapeutic concentrations, but with a single predominant mechanism of action that For topiramate, felbamate, retigabine, losigamone and stiripentol, GABA A R modulation is one of several possible antiseizure mechanisms. Allopregnanolone, a progesterone metabolite that enhances GABA A R function, led to the development of ganaxolone. Mechanism of Action Although gabapentin is a structural analog of the neurotransmitter gamma-aminobutyric acid (GABA), it appears not to interact with GABA receptors. Its mechanism of action is not entirely clear but is likely related to inhibition of calcium and, possibly, sodium channels. 1 Mechanism of Action. Gabapentin is designed as GABA analog (similar to pregabalin), which means it binds to the α2δ (alpha-2-delta) Gabapentin is an anti-epileptic agent but now it is also recommended as first line agent in neuropathic pain, particularly in diabetic neuropathy and post herpetic neuralgia. α2δ-1, an auxillary subunit of voltage gated calcium channels, has been documented as its main target and its specific binding to this subunit is described to produce different actions responsible for pain attenuation Mechanisms of action. Gabapentin and pregabalin do not bind to GABA receptors despite their structural similarity but have a high affinity for the α2δ-1 subunit of voltage-gated calcium channels (VGCCs). 19 VGCCs are composed of multiple subunits: α 1, β, γ and α 2 δ.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |