Gallery
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 |  |
 |  |
 |  |
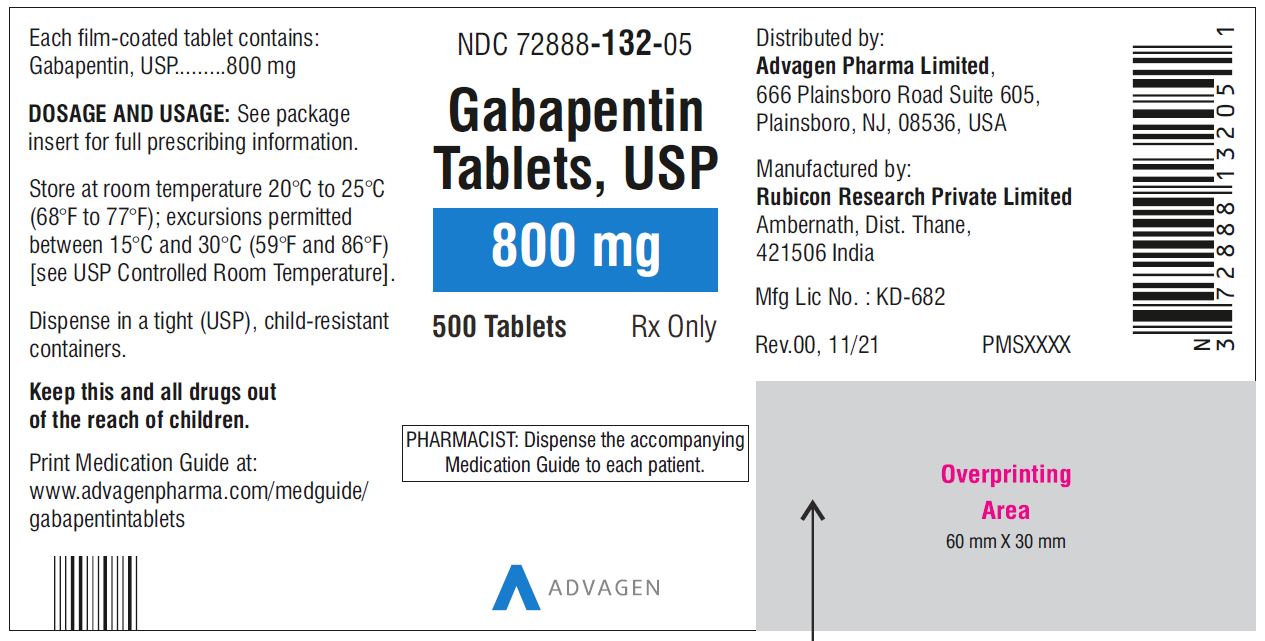
This Medication guide has been approved by the U.S. Food and Drug Administration. This product’s label may have been updated. For current full prescribing information, please visit Gabapentin, USP is a white to off-white crystalline powder. It is freely soluble in water and in alkaline and acidic solutions. The log of the partition coefficient (n-octanol/0.05M phosphate buffer) at pH 7.4 is −1.25. NEURONTIN (gabapentin) capsules, tablets, and oral solution are supplied as follows: 100 mg capsules: White hard gelatin capsules printed with "PD" on the body and "Neurontin/100 mg" on the Advise the patient to read the FDA-approved patient labeling (Medication Guide). Advise the patient to read the FDA-approved patient labeling (Medication Guide). Administration Information. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity Gabapentin is a prescription medication that was approved by the U.S. Food and Drug Administration in 1993 as a treatment for epilepsy. It works by binding to a type of calcium channel in nerve In 2004, gabapentin became available as a generic drug. No one seems to fully understand exactly how gabapentin works within our bodies, although it is deemed effective. “Gabapentin treats seizures by decreasing abnormal excitement in the brain. Gabapentin relieves the pain of PHN by changing the way the body senses pain. Antiepileptic drugs (AEDs), including gabapentin, the active ingredient in gabapentin tablet, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and Gabapentin tablets are indicated for: Patients 3 to 11 years of age: starting dose range is 10 to 15 mg/kg/day, given in three divided doses; recommended dose in patients 3 to 4 years of age is 40 mg/kg/day, given in three divided doses; the recommended dose in patients 5 to 11 years of age is 25 to 35 mg/kg/day, given in three divided doses. See full prescribing information for gabapentin capsules. INDICATIONS AND USAGE - Gabapentin capsules, USP, are indicated for: -management of postherpeticneuralgia in adults - -Adjunctive therapy in the treatment of partial onset seizures, with and DOSAGE AND ADMINISTRATION - Gabapentin capsules, USP are given orally with or without food. This label may not be the latest approved by FDA. For current labeling information, please visit particular, gabapentin prevents pain-related responses in In 1993, the FDA approval of Neurontin, the original branded gabapentin, was for use as an adjunctive medication to control partial seizures. 9 Over the next several years, the manufacturer, Parke-Davis, a subsidiary of Warner-Lambert, engaged in a large marketing campaign to increase off-label prescribing of Neurontin for pain. 4 By the mid Gabapentin is an anticonvulsant (antiseizure) medication approved by the FDA to treat several conditions. Doctors sometimes prescribe gabapentin "off-label" to treat other conditions as well. A 2022 report stated that gabapentin was among the 10 most commonly prescribed medications in the U.S. What is gabapentin and what is it used for? During the controlled epilepsy trials in patients older than 12 years of age receiving doses of Gabapentin up to 1800 mg daily, somnolence, dizziness, and ataxia were reported at a greater rate in patients receiving Gabapentin compared to placebo: i.e., 19% in drug versus 9% in placebo for somnolence, 17% in drug versus 7% in placebo for Gabapentin (Neurontin, Gralise, Horizant) is a medicine used to treat partial seizures, nerve pain from shingles and restless leg syndrome. It works on the chemical messengers in your brain and nerves. Gabapentin is from a group of medicines called anticonvulsants. Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly During the controlled epilepsy trials in patients older than 12 years of age receiving doses of gabapentin up to 1,800 mg daily, somnolence, dizziness, and ataxia were reported at a greater rate in patients receiving gabapentin compared to placebo: i.e., 19% in drug versus 9% in placebo for somnolence, 17% in drug versus 7% in placebo for The rise in gabapentin prescribing is multifactorial but thought to be due in part to efforts by the pharmaceutical industry to promote the use of the medication for off-label uses. (In 2004, the manufacturer of Neurontin, Pfizer, pleaded guilty to multiple counts of illegally promoting the off-label use of gabapentin, resulting in nearly $430 Advise the patient to read the FDA-approved patient labeling (Medication Guide). Administration Information. Inform patients that gabapentin is taken orally with or without food. Inform patients that, should they divide the scored 600 mg or 800 mg tablet in order to administer a half-tablet, they should take the unused half-tablet as the next dose. Administer NEURONTIN three times a day using 300 mg or 400 mg capsules, or 600 mg or 800 mg tablets. The maximum time between doses should not exceed 12 hours. This label may not be the latest approved by FDA. For current labeling information, please visit particular, gabapentin prevents pain-related responses in
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 |  |
 |  |
 |  |