Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
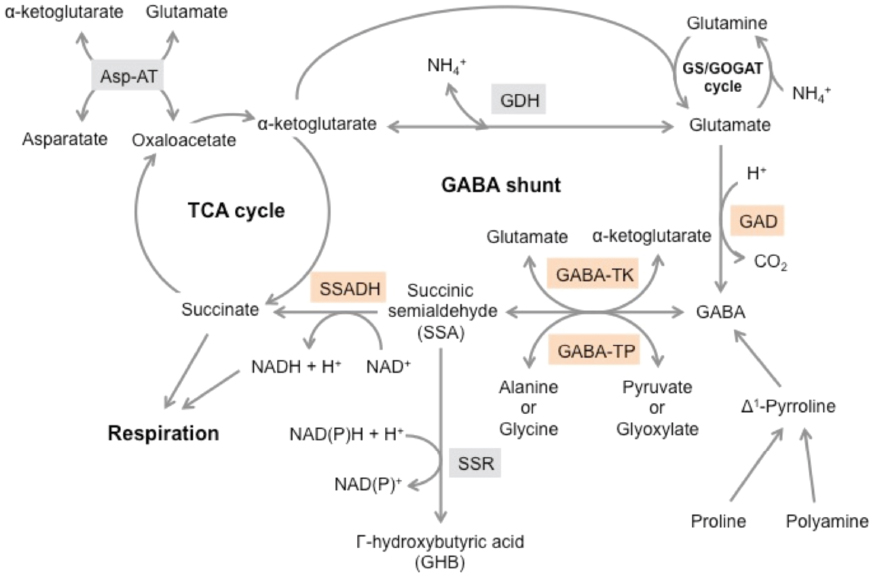
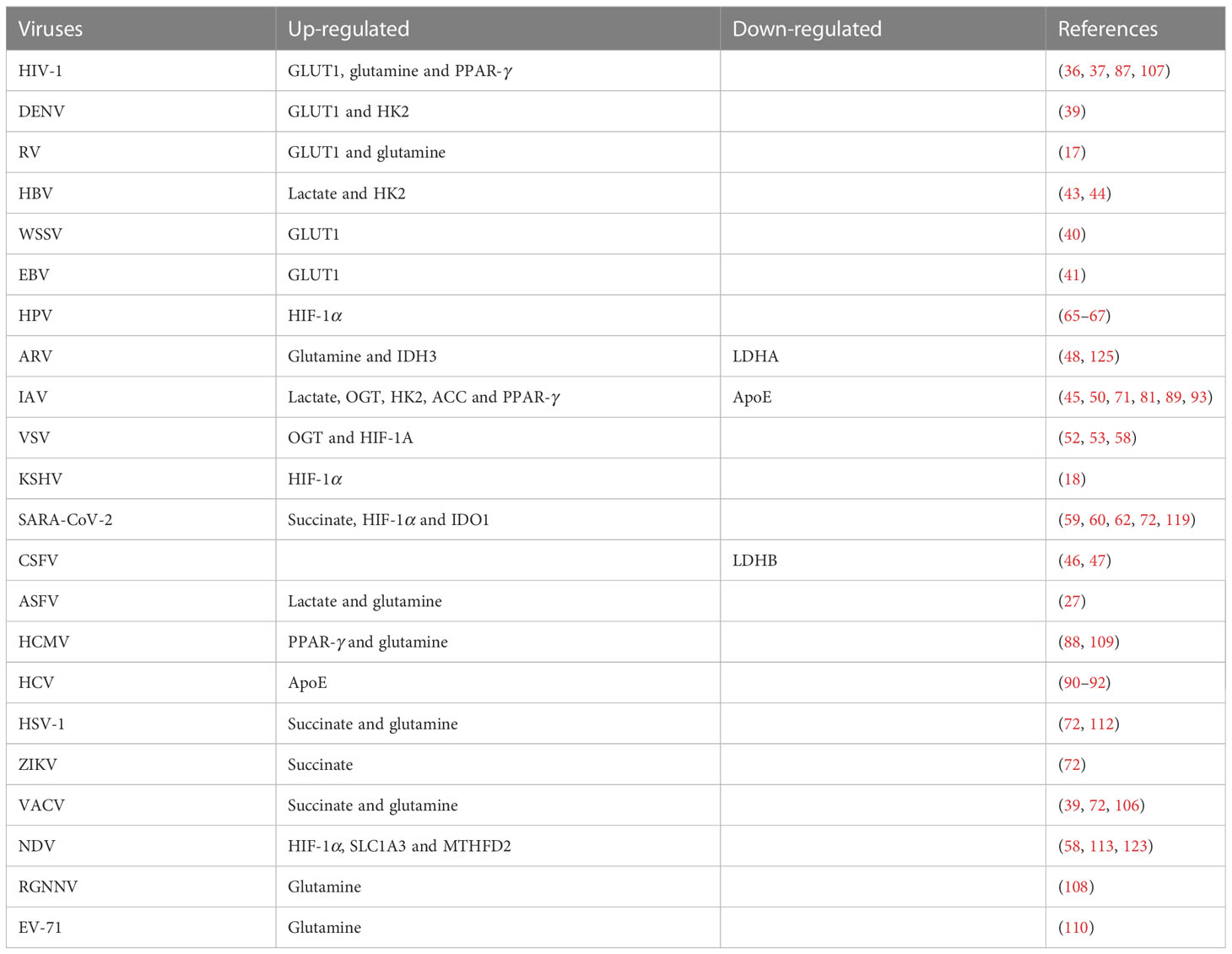
Gabapentin does not bind to other drug transporters such as P-glycoprotein (ABCB1) or OCTN2 (SLC22A5). [95] It is not significantly bound to plasma proteins (<1%). [94] Gabapentin undergoes little or no metabolism. [86] [94] Gabapentin is generally safe in people with liver cirrhosis. [99] Gabapentin is eliminated renally in the urine. [94] In addition to their use in managing neuropathic pain, gabapentinoids are increasingly being used for off-label conditions despite the lack of evidence. Prescription rates for off-label Metabolism: In humans, gabapentin undergoes minimal metabolic alteration, largely retaining the original structure. Gabapentin does not induce or inhibit CYP enzymes. Also, none of the CYP enzyme inhibitors alter their pharmacokinetics. The precise mechanism through which gabapentin exerts its therapeutic effects is unclear. 16,17 The primary mode of action appears to be at the auxillary α2δ-1 subunit of voltage-gated calcium channels (though a low affinity for the α2δ-2 subunit has also been reported). 10,8,14 The major function of these subunits is to facilitate the Gabapentin is a new antiepileptic drug (AED) with an attractive pharmacokinetic profile. It is absorbed by an active and saturable transport system, and has a high volume of distribution. Gabapentin is not bound to plasma proteins, does not induce hepatic enzymes and is not metabolized. At steady st The pharmacokinetic (PK) properties of gabapentin, including absorption, distribution, metabolism, and excretion (ADME), were investigated during the development of Neurontin®, an immediate-release (IR) formulation of gabapentin that is orally administered three-times daily. Gabapentin is effective as monotherapy for partial seizures (Beydoun 1999).However, although few studies have compared the effectiveness of gabapentin with other AEDs in the management of partial epilepsy, available evidence suggests that monotherapy with gabapentin is less effective than traditional agents such as carbamazepine and the newer antiepileptic drug lamotrigine (Marson et al. 2007). Gabapentin is a novel anticonvulsant drug. The anticonvulsant mechanism of gabapentin is not known. Based on the amino acid structure of gabapentin we explored its possible effects on glutamate and γ-aminobutyric acid (GABA) metabolism in brain as they may relate to its anticonvulsant mechanisms of action. Summary: This paper describes the pharmacokinetic studies of 1-(aminomethyl)-cyclohexane acetic acid (gabapentin, Go 3450, CI-945) conducted with the '4C-labelled substance fal-lowing intravenous and intragastric administration to rats and dogs and oral administration to humans. In man, no biotransformation of gabapentin is observed. In rats, biotransformation is only minor. In dogs, however, a remarkable formation of N-methyl-gabapentin is found. Elimination half-lives range between 2-3 h in rats, 3-4 h in dogs, and 5-6 h in man. Gabapentin is nearly exclusively eliminated via the kidneys. Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly Although both gabapentinoids are absorbed in the small intestine, pregabalin is also absorbed in the proximal colon. Absorption of gabap-entin is solely dependent on LAT that are easily satura-ble, resulting in dose-dependent pharmacokinetics. Gabapentin has no direct GABAergic action and does not block GABA uptake or metabolism. Gabapentin blocks the tonic phase of nociception induced by formalin and carrageenan, and exerts a potent inhibitory effect in neuropathic pain models of mechanical hyperalgesia and mechanical/thermal allodynia. Both can be given without regard to food intake since the amount absorbed is unaffected by food. Apparent volumes of distribution for pregabalin and gabapentin are 0.5 and 0.8 L/kg, respectively. Neither drug is metabolized, inhibits the enzymes responsible for the metabolism of other drugs, nor is bound to plasma proteins. Goldlust A, Su T-Z, Welty De Taylor CP, Oxender DL (1995) Effects of anticonvulsant drug gabapentin on the enzymes in metabolic pathways of glutamate and GABA. Epilepsy Res 22:1–11. Article PubMed CAS Google Scholar The chemical structure of gabapentin (Neurontin) is derived by addition of a cyclohexyl group to the backbone of gamma-aminobutyric acid (GABA). Gabapentin prevents seizures in a wide variety of models in animals, including generalized tonic-clonic and partial seizures. Gabapentin has no activity at Gabapentin is not protein-bound. A high volume of distribution indicates greater concentration in tissue than in plasma. It is not metabolized and does not induce hepatic enzymes or inhibit metabolism of other antiepileptic drugs. Absorption of gabapentin is solely dependent on LAT that are easily saturable, resulting in dose-dependent pharmacokinetics. As the dose of gabapentin increases, the area under the plasma concentration–time curve (AUC) does not increase proportionally. Gabapentin has been shown to influence the metabolism of neurotransmitters such as glutamate and GABA. It inhibits the cytosolic form of branched-chain aminotransferase (BCATc), which is involved in the synthesis of glutamate from branched-chain amino acids . Gabapentin is absorbed slowly after oral administration, with maximum plasma concentrations attained within 3-4 hours. Orally administered gabapentin exhibits saturable absorption--a nonlinear (zero-order) process--making its pharmacokinetics less predictable.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |