Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
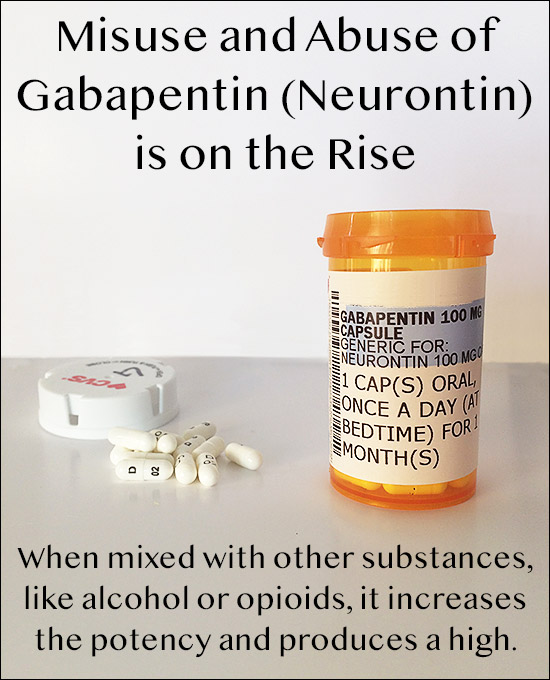 |  |
Off-label gabapentin (Neurontin) got a bad rep when it missed the mark in bipolar disorder, but there may be something worth salvaging in this drug. Here, we weigh its pros and cons for anxiety, substance use disorders, sleep, pain, and hot flashes, and compare it to its underutilized cousin, pregabalin (Lyrica). “Gabapentin is widely used in the United States for a number of off-label indications, often as an alternative to opioid therapy. Increasing evidence has emerged suggesting that gabapentin may not be as benign as once thought and may be associated with substance abuse in concert with opioids We selected gabapentin (Neurontin), a medication reported to be widely used off label, as a specific example to explore specialist physicians' experiences with off-label prescribing. Gabapentin is used to control seizures, to treat nerve pain that can happen after having had shingles, and to treat a condition called restless legs syndrome. In addition to these FDA-approved uses, doctors sometimes prescribe gabapentin off-label. Gabapentin, sold under the brand name Neurontin among others, is an anticonvulsant medication primarily used to treat neuropathic pain and also for partial seizures [10] [7] of epilepsy. It is a commonly used medication for the treatment of neuropathic pain caused by diabetic neuropathy , postherpetic neuralgia , and central pain . [ 11 ] Regardless of the settlement outcome, Gabapentin (brand name “Neurontin”) is still commonly prescribed for a variety of off-label conditions including: fibromyalgia, chronic pain, bipolar disorder, alcohol withdrawal, and migraine headaches. Brand names include Neurontin and Gralise. Tedeschi says gabapentin is also sometimes prescribed off-label by providers for a variety of other medical conditions. (Off-label use means evidence In addition to these FDA-approved uses, doctors sometimes prescribe gabapentin off-label. Off-label use means there is some evidence to show that a drug may be medically appropriate to treat conditions other than those for which it was approved. Gabapentin is sold under the brand name Neurontin and is available as a generic product as well. In December 1993, the US Food and Drug Administration (FDA) granted approval for gabapentin, under the brand name Neurontin, for adjunctive therapy of partial seizures. Subsequently, the FDA approved gabapentin in 2000 for treatment of partial seizures in children aged 3 years or older and in 2002 for treatment of postherpetic neuralgia. Background: The gabapentinoid drugs gabapentin and pregabalin were originally developed as antiseizure drugs but now are prescribed mainly for treatment of pain. . For gabapentin, the only pain-related indication approved by the US Food and Drug Administration (FDA) is postherpetic neuralg Background The gabapentinoid drugs gabapentin and pregabalin were originally developed as antiseizure drugs but now are prescribed mainly for treatment of pain. For gabapentin, the only pain-related indication approved by the US Food and Drug Administration (FDA) is postherpetic neuralgia. Two doctors recently reviewed published evidence for the benefits and risks of off-label use of gabapentin (originally sold under the trade name Neurontin) and its brand-name cousin Lyrica OBJECTIVES: (1) Describe the relevance of off-label use of gabapentin to man-aged care pharmacy; (2) summarize recent FDA warnings and media reports related to off-label gabapentin use; (3) review medical information pertaining to the off-label use of gabapentin; (4) outline alternatives to off-label use of Gabapentin is widely used in the United States for a number of off-label indications, often as an alternative to opioid therapy. Increasing evidence has emerged suggesting that gabapentin may not be as benign as once thought and may be associated with substance abuse in concert with opioids. Gabapentin was the tenth most commonly prescribed medication in the United States in 2017, and pregabalin ranked sixth in nondiscounted spending for brand-name drugs that same year (with that spending rising from $2.4 to $4.9 billion). For the evidence addressing off-label gabapentinoid use, their noteworthy findings included: Gabapentinoids, a class of medications including gabapentin (brand name Neurontin) and pregabalin (brand name Lyrica), are among the most prescribed for off-label uses. Gabapentinoids are with approved by the FDA to treat certain specific conditions, such as seizures and fibromyalgia, but 95% of prescriptions are for off-label uses such as low We examined clinical trials of gabapentin (Neurontin, Pfizer) for off-label use for migraine prophylaxis, bipolar disorders, neuropathic pain, or nociceptive pain. Outcomes described in Gabapentin is sold generically and under brand names like Neurontin, Gralise, Horizant, SmartRx Gaba-V Kit, and Neuraptine. Gabapentin was originally approved by the Food and Drug Administration (FDA) in 1993 to be used with other anti-seizure medications to control partial seizures in adults. Gabapentin enacarbil (brand name Horizant) is a prodrug of gabapentin that has been designed to overcome the limitations of gabapentin, such as poor absorption and a short duration of action. It requires hydrolyzation in the gastrointestinal tract to become active. Brand names include Neurontin and Gralise. Tedeschi says gabapentin is also sometimes prescribed off-label by providers for a variety of other medical conditions. (Off-label use means evidence
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |