Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
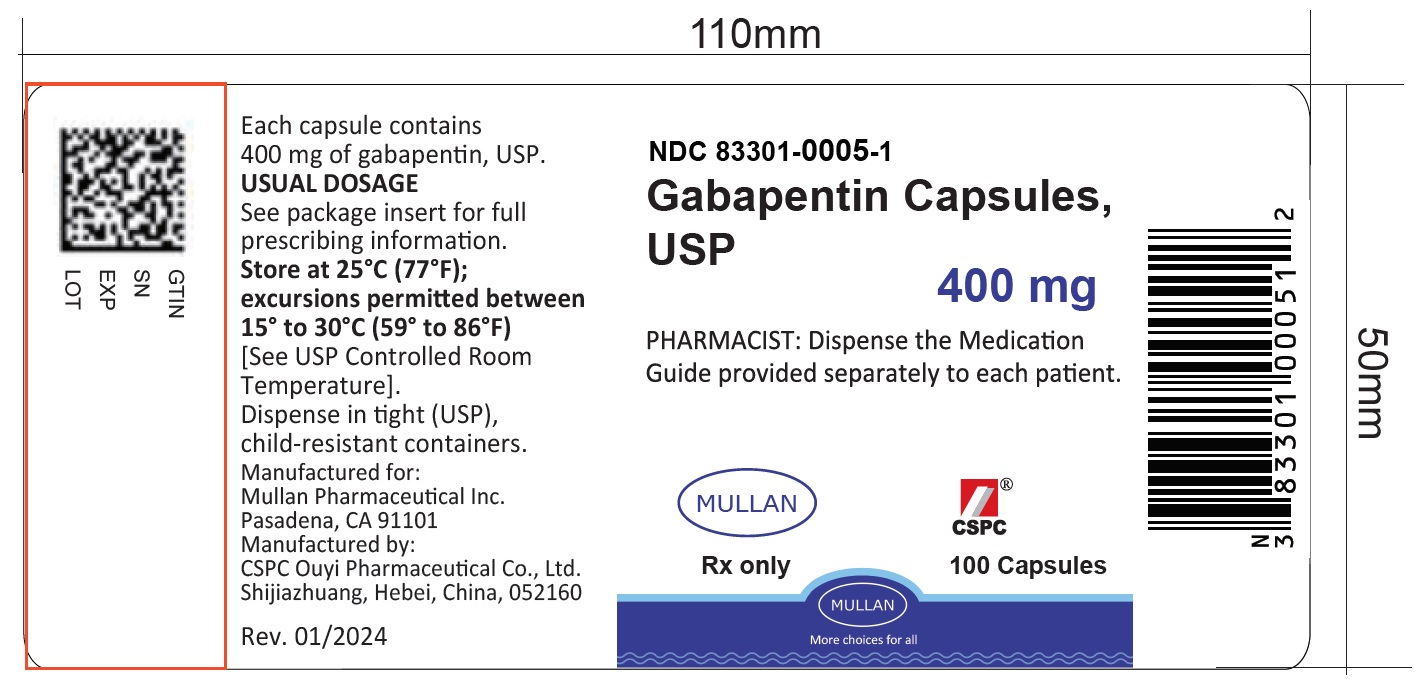
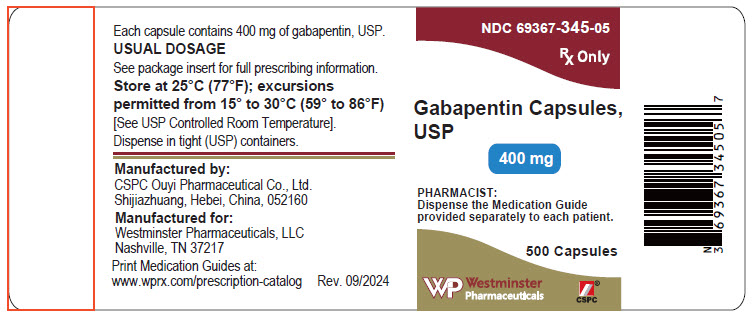
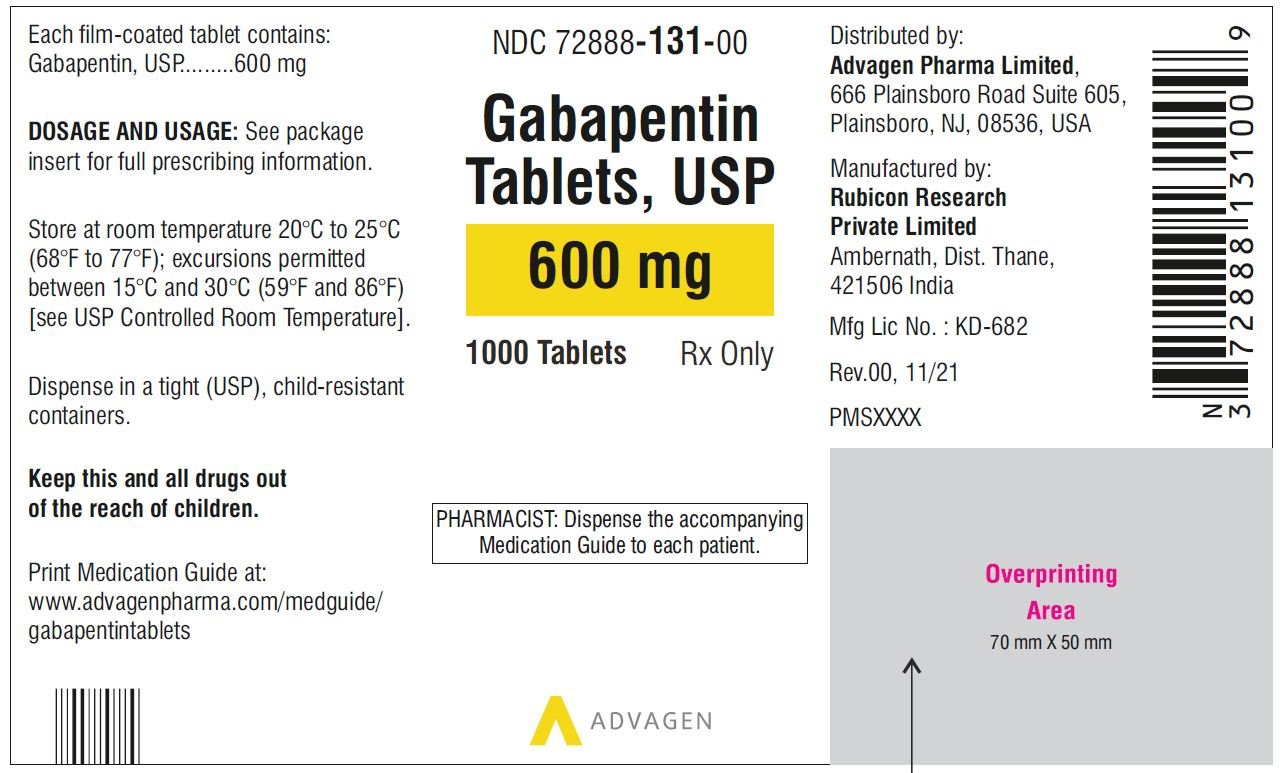
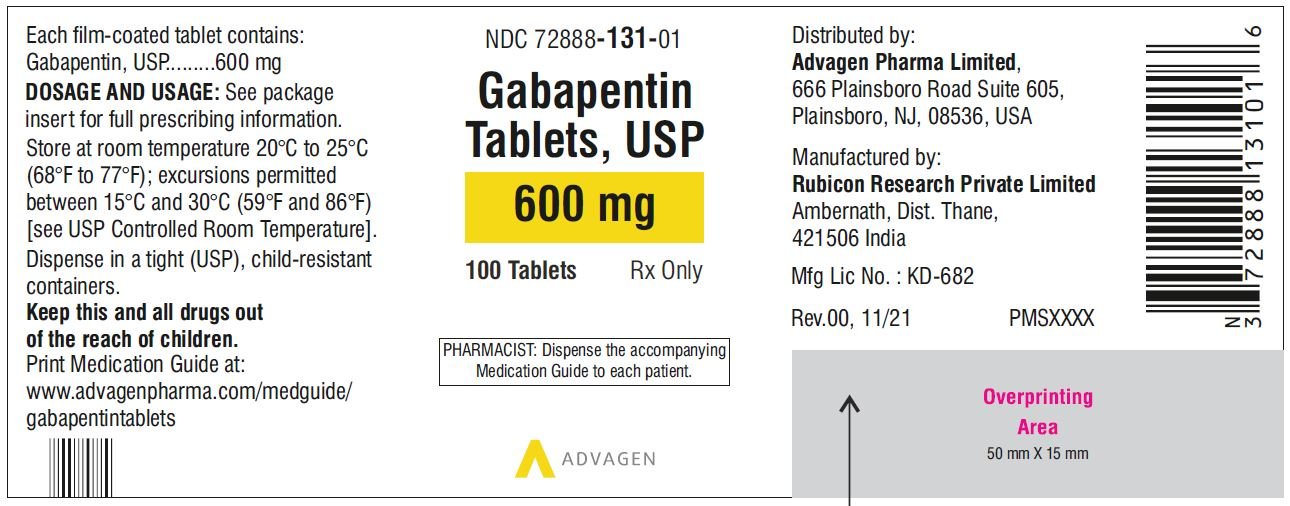
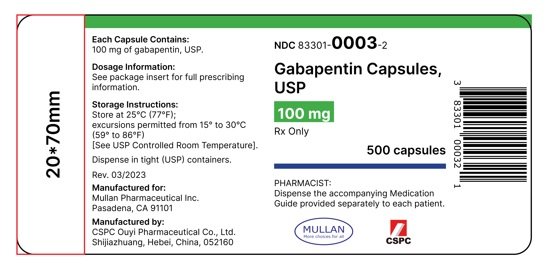
Find the dosage, indications, warnings, and side effects of gabapentin, a gamma-aminobutyric acid analog used for postherpetic neuralgia and epilepsy. See the full prescribing information and medication guide for gabapentin capsules. Gabapentin, USP is a white to off-white crystalline powder. It is freely soluble in water and in alkaline and acidic solutions. The log of the partition coefficient (n-octanol/0.05M phosphate buffer) at pH 7.4 is −1.25. maintenancedose of NEURONTIN in patients 3 to 4 years of age is 40mg/kg/day,given in three divided doses. The recommended maintenancedose of NEURONTIN in patients 5 to 11 years of age is 25mg/kg/day to 35mg/kg/day, given in three divided doses. NEURONTIN may be administered as the oral solution, capsule, or Find the description, mechanism of action, pharmacokinetics, and dosage of gabapentin, a drug used for neuropathic pain and epilepsy. The label also includes warnings, precautions, and adverse reactions of gabapentin. A literature article reported that when a 60 mg controlled-release morphine capsule was administered 2 hours prior to a 600 mg NEURONTIN capsule (N=12), mean gabapentin AUC increased by 44% compared to gabapentin administered without morphine. Administer gabapentin capsules orally with or without food. Gabapentin capsules should be swallowed whole with water. If the gabapentin capsules dose is reduced, discontinued, or substituted with an alternative medication, this should be done gradually over a minimum of 1 week (a longer period may be needed at the discretion of the prescriber). Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly This document provides the indications, dosage, administration, and warnings of gabapentin capsules, a drug used for postherpetic neuralgia and epilepsy. It also includes information on dosage adjustment, contraindications, and adverse reactions of gabapentin. This document provides the indications, dosage, administration, and warnings of gabapentin, a drug used for postherpetic neuralgia and epilepsy. It also includes information on dosage adjustment, administration, and contraindications based on renal function and age. NEURONTIN is a drug approved for postherpetic neuralgia and epilepsy. See dosage, warnings, adverse reactions, drug interactions, and other information for NEURONTIN. This web page provides the official product information for gabapentin tablets, a medication for postherpetic neuralgia and epilepsy. It includes dosage, administration, and dosage adjustment recommendations based on renal function and age. This document provides information on the indications, dosage, contraindications, warnings, adverse reactions, and drug interactions of gabapentin oral solution. It also includes patient counseling and medication guide for gabapentin oral solution. Gabarone package insert / prescribing information for healthcare professionals. Gabapentin use in pediatric patients with epilepsy 3 to 12 years of age is The recommended maintenance dose of gabapentin in patients 3 to 4 years of age is 40 mg/kg/ day, given in three divided doses. The recommended maintenance dose of gabapentin in patients 5 to 11 years of age is 25 mg/kg/day to 35 mg/kg/day, given in three divided doses. Gabapentin may Gabapentin capsules are indicated for postherpetic neuralgia and epilepsy with partial onset seizures. See dosage, contraindications, warnings, adverse reactions, and drug interactions for gabapentin capsules. Gabapentin Capsules package insert / prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions and pharmacology. Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly NEURONTIN is a drug approved for postherpetic neuralgia and epilepsy. It has warnings, precautions, adverse reactions, and dosage information. See full prescribing information for NEURONTIN. Gabapentin Tablets package insert / prescribing information for healthcare professionals. Includes: indications, dosage, adverse reactions and pharmacology. GABAPENTIN- gabapentin tablet, film coated GABAPENTIN- gabapentin suspension Greenstone LLC-----HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use GABAPENTIN safely and effectively. See full prescribing information for GABAPENTIN. Gabapentin capsules, for oral use Gabapentin tablets, for oral use
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |