Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
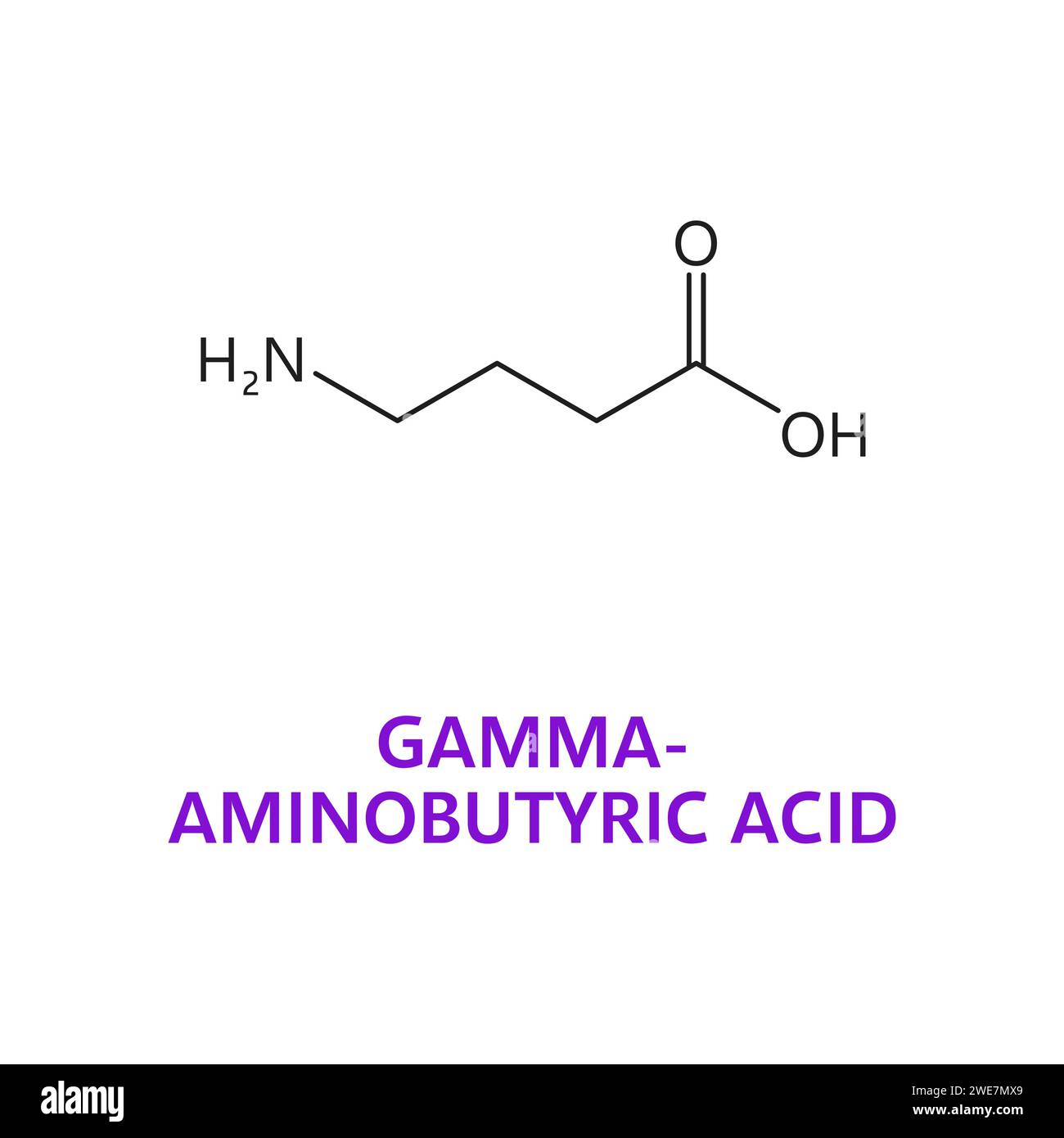 |  |
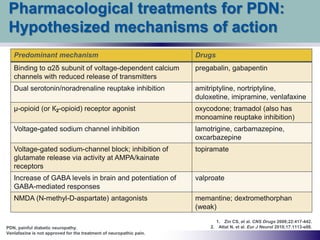
While the distribution of [3 H]gabapentin binding sites was qualitatively similar to that of the NMDA receptor, as defined by strychnine-insensitive [3 H]glycine binding, regression analysis yielded a correlation coefficient of only 0.48. Gabapentin works by showing a high affinity for binding sites throughout the brain corresponding to the presence of the voltage-gated calcium channels, especially α-2-δ-1, which seems to inhibit the release of excitatory neurotransmitters in the presynaptic area that participate in epileptogenesis. Gabapentin binding to the Ca v α 2 δ-1subunit is competitively inhibited by l -leucine, an amino acid that occupies the gabapentin-binding site without affecting calcium channel Gabapentin antagonizes thrombospondin binding to α2δ–1 and powerfully inhibits excitatory synapse formation in vitro and in vivo. These findings identify α2δ–1 as a receptor involved in excitatory synapse formation and suggest that gabapentin may function therapeutically by blocking new synapse formation. INTRODUCTION Gabapentin has no activity at GABAA or GABAB receptors of GABA uptake carriers of brain. Gabapentin interacts with a high-affinity binding site in brain membranes, which has recently been identified as an auxiliary subunit of voltage-sensitive Ca2+ channels. Gabapentin and pregabalin do not bind to GABA receptors despite their structural similarity but have a high affinity for the α2δ-1 subunit of voltage-gated calcium channels (VGCCs). 19 VGCCs are composed of multiple subunits: α 1, β, γ and α 2 δ. Despite the fact that gabapentinoids are GABA analogues, gabapentin and pregabalin do not bind to GABA receptors, do not convert into GABA Tooltip γ-aminobutyric acid or GABA receptor agonists in vivo, and do not modulate GABA transport or metabolism. Gabapentinoid drugs for pain and anxiety act on the Ca V α 2 δ-1 and Ca V α 2 δ-2 subunits of high-voltage-activated calcium channels (Ca V 1s and Ca V 2s). Here we present the cryo-EM structure of the gabapentin-bound brain and cardiac Ca V 1.2/Ca V β 3 /Ca V α 2 δ-1 channel. α2δ-1, commonly known as a voltage-activated Ca 2+ channel subunit, is a binding site of gabapentinoids used to treat neuropathic pain and epilepsy. However, it is unclear how α2δ-1 contributes to neuropathic pain and gabapentinoid actions. Gabapentin Receptor a2d-1 Is a Neuronal Thrombospondin Receptor Responsible for Excitatory CNS Synaptogenesis C¸ agla Eroglu,1,2,* Nicola J. Allen,2 Michael W. Susman,2 Nancy A. O’Rourke,3 Chan Young Park,2 Engin O¨ zkan,3,4 Chandrani Chakraborty, 2Sara B. Mulinyawe, Douglas S. Annis,5 Andrew D. Huberman, Eric M. Green,2 Jack Lawler,8 Gabapentin antagonizes thrombospondin binding to alpha2delta-1 and powerfully inhibits excitatory synapse formation in vitro and in vivo. These findings identify alpha2delta-1 as a receptor involved in excitatory synapse formation and suggest that gabapentin may function therapeutically by blocking new synapse formation. The approximate position of the von Willebrand factor A domain (required for trafficking of α 1 subunit of VGCCs (Canti et al. 2005)) is shown in close proximity to gabapentin and pregabalin-binding site in α 2 δ-1 and α 2 δ-2 (RRR). However, receptor binding studies fail to detect any specific interactions between gabapentin and the NMDA receptor complex , including the strychnine-insensitive glycine site . In the absence of specific binding data, it is unlikely that gabapentin has its effect by means of interacting with the NMDA receptor. Originally designed as analogs of GABA, the gabapentinoids bind to the α 2 δ ‐1 and α 2 δ ‐2 auxiliary subunits of calcium channels, though only the former has been implicated in the development of neuropathy in animal models. Generic Name Gabapentin DrugBank Accession Number DB00996 Background. Gabapentin is a structural analogue of the inhibitory neurotransmitter gamma-aminobutyric acid that was first approved for use in the United States in 1993. 16 It was originally developed as a novel anti-epileptic for the treatment of certain types of seizures 14,5 - today it is also widely used to treat neuropathic pain. 8 Gabapentin's binding site is located on the α 2 δ subunit of voltage gated calcium channels. 6 In vitro, gabapentin can inhibit neuronal calcium currents by 35% and decrease neuronal tachykinin-mediated activity. 7,8 It has been suggested that mitigation of hypothalamic tachykinin neurotransmitter activity via a decrease in neuronal calcium Gabapentin antagonizes thrombospondin binding to α2δ-1 and powerfully inhibits excitatory synapse formation in vitro and in vivo. These findings identify α2δ-1 as a receptor involved in excitatory synapse formation and suggest that gabapentin may function therapeutically by blocking new synapse formation. The NMDA receptor complex has a number of binding sites for various ligands that regulate its activity, including the strychnine-insensitive glycine binding site, phencyclidine binding site, polyamine binding site, redox modulatory site and a proton-sensitive site. This study suggested that the antiepileptic GABA analogue gabapentin (Neurontin) is an agonist at GABA(B) receptors expressing the GABA(B1a) but not the GABA(B1b) receptor subunit. The importance of this finding with respect to identifying novel GABA(B) receptor subunit specific agonists prompted us to repeat these experiments in our own [35S Gabapentin binding to the Cavα2δ-1subunit is competitively inhib-ited by l-leucine, an amino acid that occupies the gabapentin-binding site without affecting calcium channel trafficking, and
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |