Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
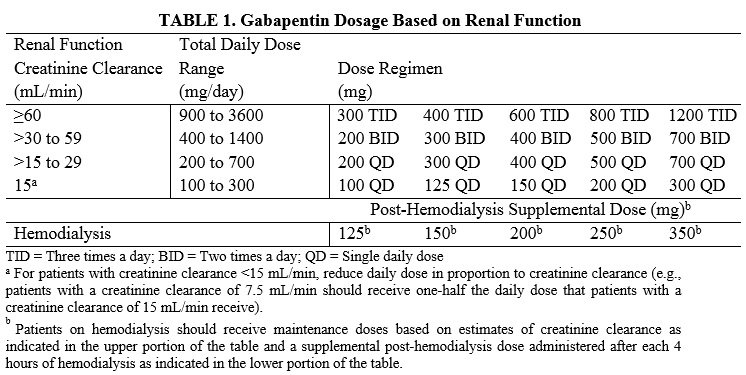
(a) Schedule V shall consist of the drugs and other substances, by whatever official name, common or usual name, chemical name, or brand name designated, listed in this section. (a) Schedule V shall consist of the drugs and other substances, by whatever official name, common or usual name, chemical name, or brand name designated, listed in this section. In 2017 and 2018, Kentucky, Tennessee, and West Virginia passed laws classifying gabapentin as a Schedule V drug due to abuse potential, risk of overdose, and death. 24–28 In contrast, between 2016 and 2018, Kansas, Massachusetts, Minnesota, Nebraska, New Jersey, North Dakota, Ohio, Virginia, and Wyoming required gabapentin to be included in Gabapentin is not currently listed as a controlled substance under federal law. However, some states classify gabapentin as a Schedule V substance or a drug of concern and mandate reporting to PMP. Furthermore, other states are considering similar actions due to increasing evidence of associated risks. As of January 1, 2023 both physician assistants (PA) and APRNs will have the same prescriptive authority. Per §30-3E-3 and §30-7-15E, the new prescriptive authority for APRNs and PAs is as below: Lost or Stolen Controlled Substances. As required by § 15-2-9.3.1: WV BOARD OF VETERINARY MEDICINE LIST OF CONTROLLED DRUGS LISTED FOR WV BUT NOT FEDERAL Xylazine ‐ WV Schedule IV Fioricet (butalbital/acetaminophen/caffeine) ‐ WV §60A-2-206. Schedule II. (a) Schedule II consists of the drugs and other substances, by whatever official name, common or usual name, chemical name or brand name designated, listed in this section. WV BOARD OF VETERINARY MEDICINE LIST OF CONTROLLED DRUGS LISTED FOR WV BUT NOT FEDERAL Xylazine ‐ WV Schedule IV Fioricet (butalbital/acetaminophen/caffeine) ‐ WV Gabapentin grew to 64 million prescriptions in the United States in 2016 from 39 million prescriptions in 2012, 12 making it the 10th most prescribed medication that year. 13 Gabapentin has been widely recognized as a drug related to opioid abuse in West Virginia. 14 Studies have shown that gabapentin in toxicology reports constituted a Schedule IV shall consist of the drugs and other substances, by whatever official name, common or usual name, chemical name, or brand name designated, listed in this section. FALSE STATEMENTS CONCERNING ANY QUESTION ON THIS APPLICATION MAY BE SUBJECT TO DISCIPLINARY ACTION INCLUDING, BUT NOT LIMITED TO, IMMEDIATE REVOCATION OR SUSPENSION OF LICENSE OR REGISTRATION. In seven states, gabapentin is classified as a schedule V controlled substance (including AL, KY, MI, ND, TN, VA, and WV). Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). The West Virginia Board of Pharmacy is continually monitoring problematic prescribing and dispensing. The passage of SB 273, which limits the day supply of initial prescriptions, will potentially help decrease the number of opioid prescriptions being dispensed in West Virginia. Gabapentin began being tracked in the CSMP in 2016 as it has become a Of those cases, gabapentin was the primary cause of death in 23 individuals. According to this report, gabapentin was amongst one of the . Of the 154 analytes detected, gabapentin accounted for 25 samples. Total exposure calls as a result of gabapentin stayed largely the same between 2017 and in 2017 and 21,423 in 2020. Among cases The West Virginia Controlled Substances Monitoring Program (CSMP) is a central repository, maintained by the West Virginia Board of Pharmacy, for collected data related to the prescription and dispensing of all Schedule II, II and IV controlled substances. As required by §60A-9-5, this report is intended to give a brief history of the Eight states have made gabapentin a schedule V controlled substance. And 12 other states require stricter reporting on gabapentin prescriptions. If you have a prescription for gabapentin, it’s best to take the lowest dose possible. All licensees who dispense Schedule II, III, IV and V controlled substances, along with opioid antagonists, to residents of West Virginia must provide the dispensing information to the West Virginia Board of Pharmacy each 24-hour period. §60A-2-210. Schedule IV. (a) Schedule IV shall consist of the drugs and other substances, by whatever official name, common or usual name, chemical name, or brand name designated, listed in this section. Prior to issuing a prescription for a Schedule II opioid drug, a provider shall: (1) advise the patient regarding the quantity of the Schedule II opioid drug and a patient’s option to fill the prescription in a lesser quantity; and (2) inform the patient of the risks associated with the Schedule II opioid drug prescribed. scheduling of gabapentin and (2) gabapentin inclusion in the state PDMP using a control group of similar states. The Schedule V change for gabapentin was implemented in three states including Kentucky, Tennessee, and West Virginia (Table 1). These three states were all among the top quartile for opioids prescribed per 100 persons according to
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |