Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
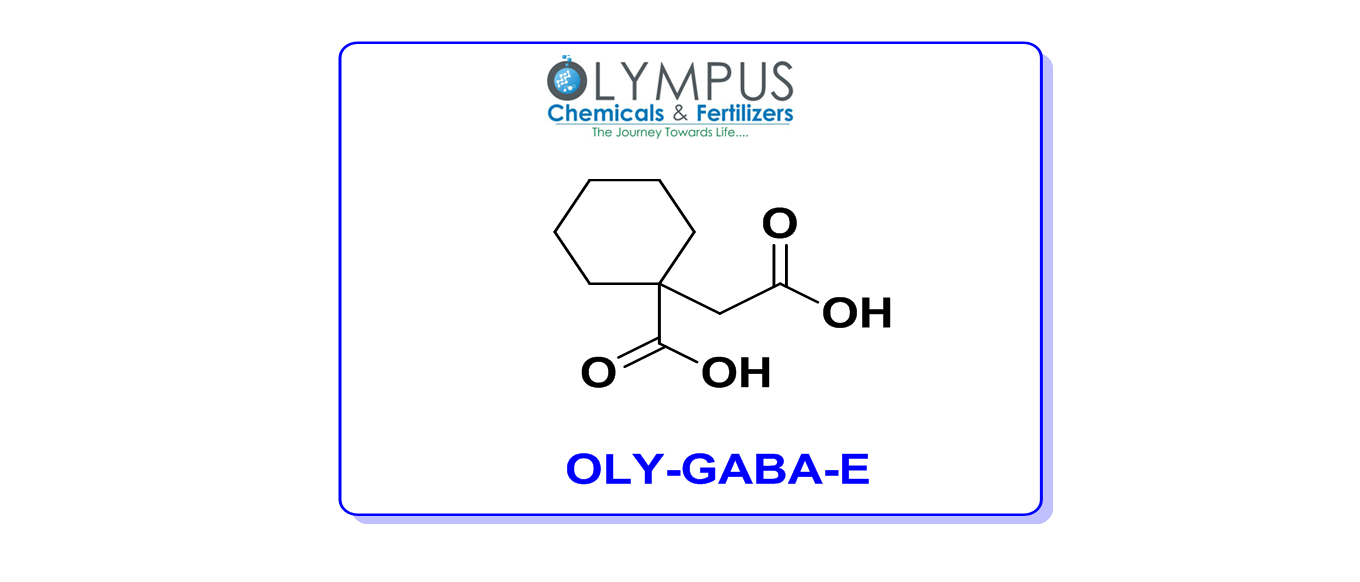
Gabapentin Capsules USP and Gabapentin Tablets USP (gabapentin) are indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. Geriatrics (>65 years of age) Systematic studies in geriatric patients have not been conducted. (See WARNINGS AND Generic brands of gabapentin capsules, USP are used for postherpetic nerve pain and for add on therapy for partial onset seizures in patients 3 years and older. Gabapentin can cause life-threatening breathing problems, especially if you already have a breathing disorder or if you use other medicines that can make you drowsy or slow your breathing. USP recommends you contact your country competent authorities to determine if any certifications, permits or licenses may be required prior to ordering. Material Origins are found within the Product under Origin Information. Store gabapentin capsules at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Keep gabapentin capsules and all medicines out of the reach of children. Gabapentin tablets USP are supplied as oval shaped, film-coated, biconvex scored tablets containing 600 mg and 800 mg of gabapentin USP. The inactive ingredients for the tablets are corn starch, copovidone, poloxamer 407, magnesium stearate, polyethylene glycol, talc, hypromellose, titanium dioxide, macrogol, polysorbate 80 and purified water. Gabapentin Capsules USP and Gabapentin Tablets USP (gabapentin) are indicated as adjunctive therapy for the management of patients with epilepsy who are not satisfactorily controlled by conventional therapy. The active ingredient in Gabapentin Capsules USP is gabapentin, which has the chemical name 1-(aminomethyl)cyclohexaneacetic acid. The molecular formula of gabapentin is C 9 H 17 NO 2 and the molecular weight is 171.24. The structural formula of gabapentin is: Gabapentin is a white to off-white crystalline solid with a pK a1 of 3.7 and a pK a2 Buy Gabapentin Related Compound E USP compendial standard (CAS 67950-95-2) to determine strength, quality, purity and identity in your USP-NF monograph tests and assays. 3296Gabapentin / Official Monographs USP 35 Gabapentin RS, USP Gabapentin Related Compound A RS, and.Gabapentin USP Gabapentin Related Compound B RS, respectively. Test solution—Use the Assay preparation. Standard solution—Dissolve a suitable quantity of USP Gabapentin Related Compound E RS in Diluent to obtain a solu- System suitability solution— Dissolve a suitable quantity of USP Gabapentin RS in Diluent, and add an appropriate volume of Impurities solution to obtain a solution containing about 14.0 mg per mL, 0.014 mg per mL, and 0.0084 mg per mL of USP Gabapentin RS, USP Gabapentin Related Compound A RS, and USP Gabapentin Related Compound B RS, respectively. Gabapentin Capsules contain NLT 90.0% and NMT 110.0% of the labeled amount of gabapentin (C 9 H 17 NO 2). Gabapentin capsules, USP are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin, USP. The inactive ingredients for the capsules are corn starch, gelatin, magnesium stearate, mannitol, sodium lauryl sulphate, talc, titanium dioxide, black edible ink which contains iron oxide black, potassium hydroxide Gabapentin USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia. Also, for use with USP monographs such as: Gabapentin Capsules CAS: 60142-96-3 Gabapentin USP / EP is a white or almost white, crystalline powder. It is soluble in water, in dilute acids and dilute solutions of alkali hydroxides. Standard preparation— Dissolve an accurately weighed quantity of USP Gabapentin RS in Diluent, and dilute quantitatively, and stepwise if necessary, with Diluent to obtain a solution having a known concentration of about 4.0 mg per mL. Gabapentin Capsules, USP are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of gabapentin. The inactive ingredients for the capsules are magnesium stearate, pregelatinized starch, starch and talc. The 100 mg capsule shell contains gelatin, sodium lauryl sulfate and titanium dioxide. USP Working standard solutions, Test solution, Chromatographic Gabapentin Related Compound A RS. system, and Procedure— Proceed as directed for Test 1 . Time: 30 minutes. Gabapentin contains NLT 98.0% and NMT 102.0% of gabapentin (C 9 H 17 NO 2), calculated on the anhydrous basis. GABAPENTIN - gabapentin tablet, film coated Sun Pharmaceutical Industries Limited -----Gabapentin Tablets, USP DESCRIPTION Gabapentin tablets, USP are supplied as elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin USP. The inactive ingredients for the tablets are glyceryl behenate, hydroxypropyl cellulose, low substituted Gabapentin Compounded Oral Suspension contains NLT 90.0% and NMT 110.0% of the labeled amount of gabapentin (C 9 H 17 NO 2).
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |