Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
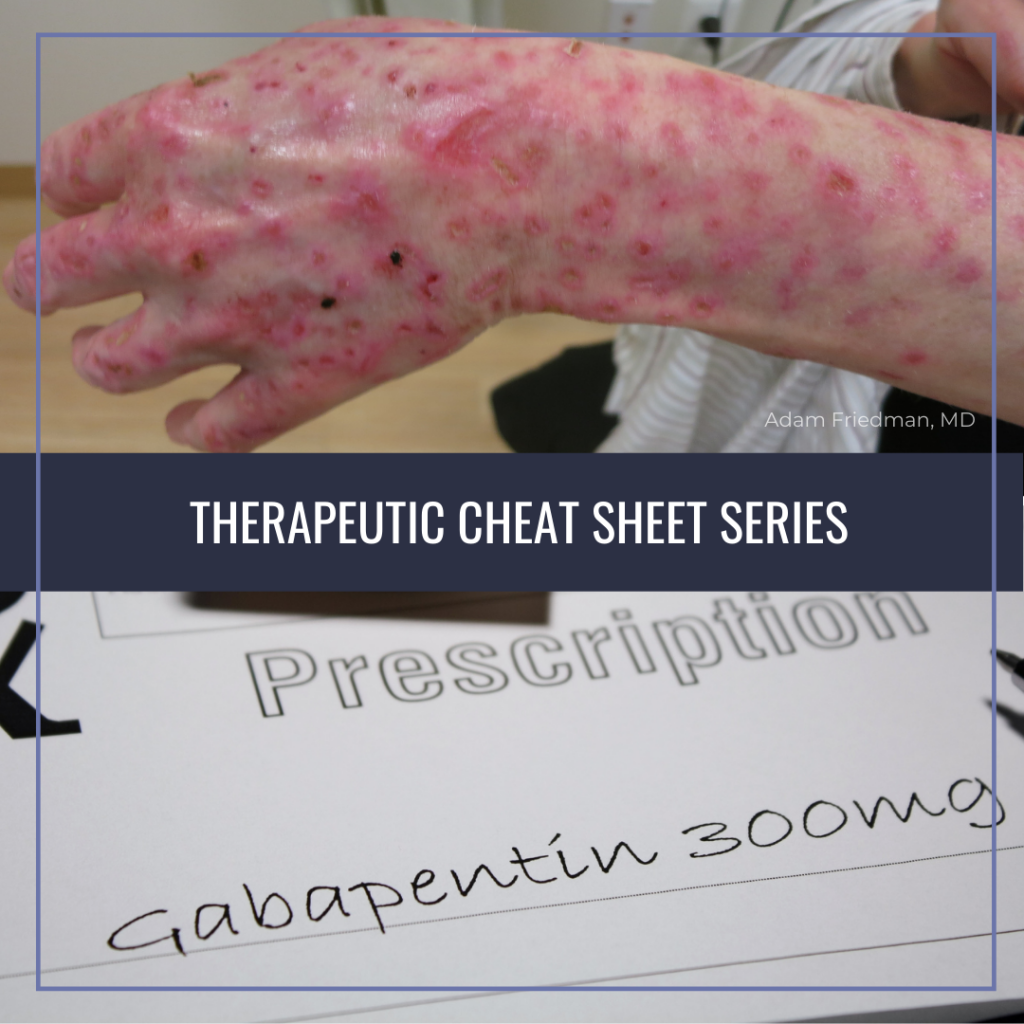
45 Antiepileptic drugs (AEDs), including gabapentin, the active ingredient in GRALISE, 46 increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Similar to gabapentin IR, the recommended total daily dose of Gralise® (gabapentin) is 1,800 mg. Both formulations require a titration schedule to achieve the effective maintenance dosage; however, with Gralise® (gabapentin) the period of titration should occur over 14 days compared to three days with gabapentin IR. Compare Gabapentin vs Gralise head-to-head with other drugs for uses, ratings, cost, side effects and interactions. Gabapentin (Neurontin, Gralise, Horizant) is a medicine used to treat partial seizures, nerve pain from shingles and restless leg syndrome. It works on the chemical messengers in your brain and nerves. Gralise (gabapentin) is used to treat post-herpetic neuralgia. Includes Gralise side effects, interactions and indications. Page 2 of 24 1 FULL PRESCRIBING INFORMATION 2 . GRA-004-C.5 SEP 2012 3 GRALISE® (gabapentin) Tablets 4 . 1 INDICATIONS AND USAGE 5 . GRALISE is indicated for the management of postherpetic neuralgia. What Is Gralise? Gralise (gabapentin) is a pain reliever used to treat pain and burning from peripheral neuropathy, and postherpetic neuralgia, a complication of shingles. What Are Side Effects of Gralise? Gralise may cause serious side effects including: hives, difficulty breathing, swelling of your face, lips, tongue, or throat, skin rash, Gralise (gabapentin) is FDA-approved for treating nerve pain from shingles in adults. It belongs to the drug class called antiepileptics. Gralise (gabapentin) is an extended-release form of gabapentin, which means that the medication gets slowly released into the body from the tablet. PHN treatment - GRALISE (gabapentin) tablets. PHARMACOKINETIC DATA DO NOT PREDICT EFFICACY OR SAFETY When GRALISE (1800 mg once daily) and gabapentin immediate release (600 mg three times a day) were administered with high-fat evening meals (50% of calories from fat), GRALISE had a higher C max and lower AUC at steady state compared to gabapentin immediate release. Gabapentin is commonly used to treat and prevent seizures in people with epilepsy or to treat nerve pain (postherpetic neuralgia) that can occur after a viral infection called shingles. GRALISE is a gastroretentive form of gabapentin for the treatment of postherpetic neuralgia (PHN). Learn how to adjust the dose based on renal function, and the safety and efficacy of GRALISE in patients with PHN. GRALISE ® (gabapentin) is a prescription medicine used in adults, 18 years and older, to treat pain from damaged nerves (neuropathic pain) that follows healing of shingles (a painful rash that comes after a herpes zoster infection). GRALISE ® (gabapentin) tablets, for oral use Initial U.S. Approval: 1993 INDICATIONS AND USAGE GRALISE is indicated for the management of Postherpetic Neuralgia (PHN). Take GRALISE exactly as your healthcare provider tells you. GRALISE tablets are available in multiple convenient strengths for tailored dosing 1. During the first 2 weeks, your healthcare provider will slowly increase your daily dose Key takeaways: Horizant and Gralise are gabapentin medications that last longer in the body than immediate-release gabapentin. These products are effective for nerve pain from shingles (Horizant and Gralise) and restless leg syndrome References: GRALISE. Prescribing information. Almatica Pharma LLC; 2023. Irving GA, Sweeney M. Tolerability and safety of gastroretentive once-daily gabapentin tablets for the treatment of postherpetic neuralgia. Medscape - Seizure dosing for Neurontin, Gralise (gabapentin), frequency-based adverse effects, comprehensive interactions, contraindications, pregnancy & lactation schedules, and cost information. At Optum, we help create a healthier world, one insight, one connection, one person at a time. All Optum trademarks and logos are owned by Optum, Inc., in GRALISE is a gabapentin product indicated for postherpetic neuralgia. It has warnings, precautions, dosage adjustments, and drug interactions related to its pharmacokinetic profile and antiepileptic effects. ADP score reduction for GRALISE was -2.1 vs -1.6 with placebo (P=0.013). 2Statistically significant pain reduction vs placebo beginning Week 1 and continuing throughout 10-week study. 2
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |