Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
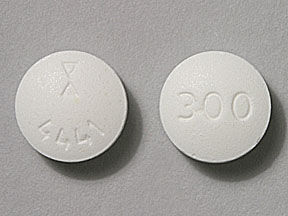 |
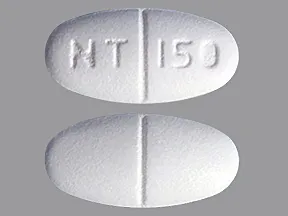
Generic Name Gabapentin DrugBank Accession Number DB00996 Background. Gabapentin is a structural analogue of the inhibitory neurotransmitter gamma-aminobutyric acid that was first approved for use in the United States in 1993. 16 It was originally developed as a novel anti-epileptic for the treatment of certain types of seizures 14,5 - today it is also widely used to treat neuropathic pain. 8 Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Healthcare professionals should evaluate patients carefully for a history of drug abuse before prescribing gabapentin, and observe patients for signs of abuse and dependence. Gabapentin isn’t a narcotic or federally controlled substance, but it is regulated and recognized as a controlled substance in certain states. Gabapentin is approved by the Food and Drug What about schedule 3 CDs? You may have noticed I skipped schedule 3 That’s because schedule 3 is complicated. It’s a kind of mixed schedule containing almost 50 controlled drugs. The rules state that about half of the schedule 3 CDs need to be kept in a CD cabinet, but the other half do not need to be locked up. In controlled clinical epilepsy trials in pediatric patients 3 to 12 years of age, the incidence of these adverse reactions was: emotional lability 6% (gabapentin-treated patients) versus 1.3% (placebo-treated patients); hostility 5.2% versus 1.3%; hyperkinesia 4.7% versus 2.9%; and thought disorder 1.7% versus 0%. Neurontin: Treats pain from shingles (postherpetic neuralgia) and, when combined with other seizure medications, treats partial-onset seizures in adults and children over 3 years old. Gralise: Specifically for postherpetic neuralgia (pain after shingles) and should not be used for any other conditions. Gabapentin isn’t considered a controlled substance by the federal government. But several states have passed their own laws limiting the prescribing and sale of it. Eight states have made gabapentin a schedule V controlled substance. Gabapentin, sold under the brand name Neurontin among others, is an anticonvulsant medication primarily used to treat neuropathic pain and also for partial seizures [10] [7] of epilepsy. It is a commonly used medication for the treatment of neuropathic pain caused by diabetic neuropathy, postherpetic neuralgia, and central pain. [11] As of 1 April 2019, pregabalin and gabapentin are controlled under the Misuse of Drugs Act 1971 as Class C substances and scheduled under the Misuse of Drugs Regulations 2001 as Schedule 3. Gabapentin – or Neurontin – is a medication commonly used to treat nerve pain and seizures. However, the drug can have potentially harmful effects when combined with other opioids. Michigan joins a growing number of states that have scheduled Gabapentin as a controlled substance. Gabapentin (Neurontin, Gralise, Horizant) is a medicine used to treat partial seizures, nerve pain from shingles and restless leg syndrome. It works on the chemical messengers in your brain and nerves. Gabapentin is from a group of medicines called anticonvulsants. Drug Schedules Drugs, substances, and certain chemicals used to make drugs are classified into five (5) distinct categories or schedules depending upon the drug’s acceptable medical use and the drug’s abuse or dependency potential. The abuse rate is a determinate factor in the scheduling of the drug; for example, Schedule I drugs have a high potential for abuse and the potential to create 3,4 cis or trans tetrahydrocannabinol, and its optical isomers; (Since nomenclature of these substances is not internationally standardized, compounds of these structures, regardless of numerical Gabapentin Conventional (immediate-release) Capsules and Tablets. 25°C (may be exposed to 15–30°C). Gabapentin Conventional (immediate-release) Oral Solution. 2–8°C. Gabapentin Gastroretentive Tablets (Gralise) 25°C (may be exposed to 15–30°C). Gabapentin Enacarbil Extended-release Tablets (Horizant) 25°C (may be exposed to 15–30 Gabapentin is a prescription drug most commonly prescribed to relieve nerve pain following shingles in adults, treating the pain of post herpetic neuralgia. Gabapentin belongs to a class of drugs known as anti- seizure drugs. Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Healthcare professionals should evaluate patients carefully for a history of drug abuse before prescribing gabapentin, and observe patients for signs of abuse and dependence. Gabapentin belongs to a class of drugs called anticonvulsants. These drugs are used to treat a variety of conditions caused by abnormal electrical activity in the brain, including seizures, nerve pain, and restless leg syndrome. Prescription drugs pregabalin and gabapentin are to be reclassified as class C controlled substances from next April, the government announced today (15 October). Gabapentin is approved to prevent and control partial seizures, relieve postherpetic neuralgia after shingles and moderate-to-severe restless legs syndrome. Learn what side effects to watch for, drugs to avoid while taking gabapentin, how to take gabapentin and other important questions and answers.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |