Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 |  |
 |  |
 |  |
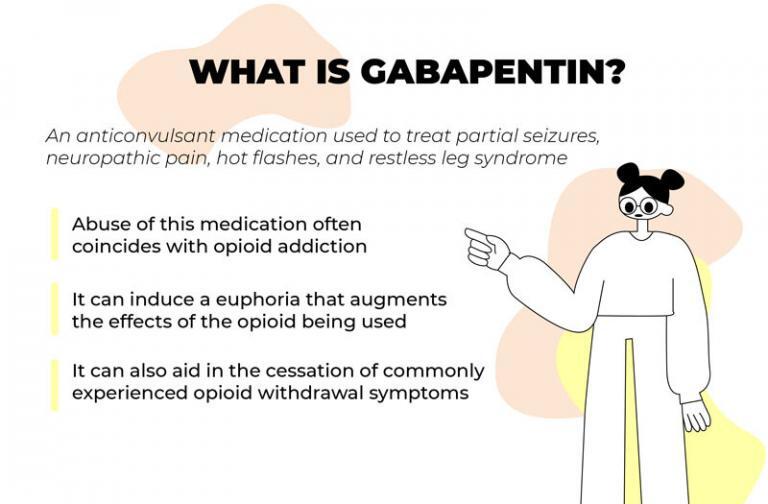
The PDMP collects data on Federally Scheduled II, III, and IV controlled substances, State Scheduled drugs including pseudoephedrine, state drugs of interest gabapentin and naloxone. The Oregon PDMP maintains this data for 3 years from pharmacy fill date. Gabapentin isn’t a controlled substance according to the federal government. But several states have passed their own laws classifying gabapentin a schedule V (schedule 5) controlled substance. Combining gabapentin and opioids can be extremely dangerous. Gabapentin is not currently listed as a controlled substance under federal law. However, some states classify gabapentin as a Schedule V substance or a drug of concern and mandate reporting to PMP. Furthermore, other states are considering similar actions due to increasing evidence of associated risks. Oregon Administrative Rules Division 80, Schedule of Controlled Substances. Refreshed: 2021-06-08 this is a joint statement from the oregon medical board and the oregon board of pharmacy regarding electronic prescriptions for controlled substances. The mission of the Oregon Medical Board is to protect the health, safety, and wellbeing of Oregon citizens by regulating the practice of medicine in a manner that promotes access to quality care. Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. The following is a list of resources to provide the most up-to-date information on laws and rules governing the practice of pharmacy and controlled substances: Oregon Revised Statutes - Chapter 689 Oregon Revised Statutes - Chapter 475 that are classified in schedules II through IV under the federal Controlled Substances Act, 21 U.S.C. 811 and 812, as modified by the board by rule under ORS 475.035; (B) Prescribed gabapentin and naloxone dispensed by pharmacies; and Twelve states have not classified gabapentin as a controlled substance, but require gabapentin dispensing must be reported to their PMP (including CT, DC, IN, KS, MA, MN, NE, NJ, OH, OR, UT, and WY). Pharmacies submit prescription data to the PDMP system for all Schedules II, III and IV controlled substances, gabapentin, and naloxone dispensed from Oregon pharmacies and to Oregon residents from non-resident pharmacies. The protected health information is collected and stored securely. The Drug Enforcement Administration (DEA) advises veterinarians to not provide their DEA license number for non-controlled substance prescriptions. Pharmacies that dispense from a veterinarian’s prescription should instead request the veterinarian’s license number. Gabapentin is not currently listed as a controlled substance under the Controlled Substances Act of 1970. 11 Several state boards of pharmacy, as outlined in Supplemental Table 2 and Figure 1, have independently reclassified gabapentin under state pharmacy rules as a Schedule V drug. Other states have required gabapentin use to be monitored To prescribe controlled substances, practitioners must obtain a DEA registration by submitting DEA Form 224 and paying a registration fee. Oregon also requires prescribers to enroll in the Prescription Drug Monitoring Program (PDMP), a state-run database tracking controlled substance prescriptions to reduce misuse. APRNs shall not personally destroy controlled substances. (e) Controlled substances must be transported in a secured, locked container. (f) Client records shall state the distribution of controlled substance samples. (g) Theft of controlled substances shall be immediately reported upon discovery to the DEA and to any other required authorities. (h) Some neurologists contend the effort to classify gabapentin as a Federal Schedule V Controlled Substance is misguided and note that it could create barriers to care for patients with neuropathic pain. schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. The chemical structure of gabapentin is derived from the addition of a lipophilic cyclohexyl group to the backbone of gamma-aminobutyric acid (GABA). Gabapentin is a crystalline substance and freely soluble in water, alkaline and acidic Gabapentin isn’t a narcotic or federally controlled substance, but it is regulated and recognized as a controlled substance in certain states. Gabapentin is approved by the Food and The Oregon PDMP collects data on Schedules II, III and IV controlled substances and gabapentin. Beginning Jan 2025, the PDMP will begin collecting schedule V drugs and veterinarian prescribed controlled substances. At the national level, gabapentin is not classified as a controlled substance under the Controlled Substances Act (CSA). This means it is not subject to the stringent regulations that apply to opioids or benzodiazepines, which are categorized based on their potential for abuse, medical use, and safety. 855-080-0020 Schedules Pursuant to ORS 475.005(6) those drugs and their immediate precursors classified in Schedules I through V under the Federal Controlled Substances Act, 21 USC 811 (03/23/2024), 21 USC 812 (03/23/2024) and as amended by the board pursuant to ORS 475.035 are the controlled substances for purposes of regulation and control under the Act.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 |  |
 |  |
 |  |
 |  |