Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 | |
 |  |
 |  |
 |  |
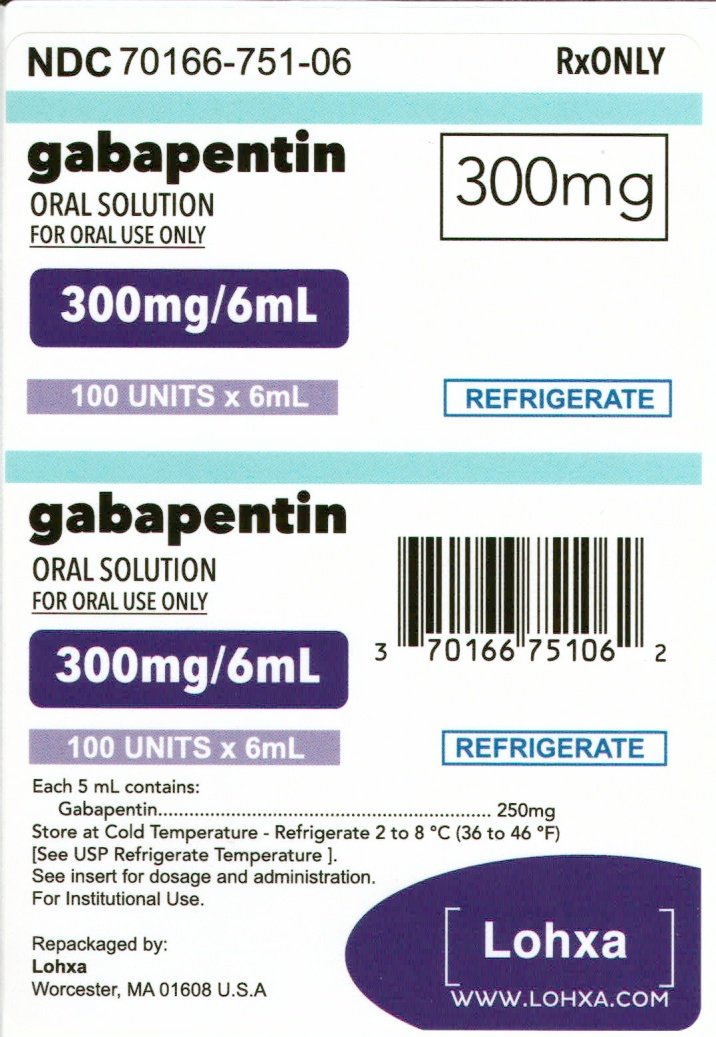
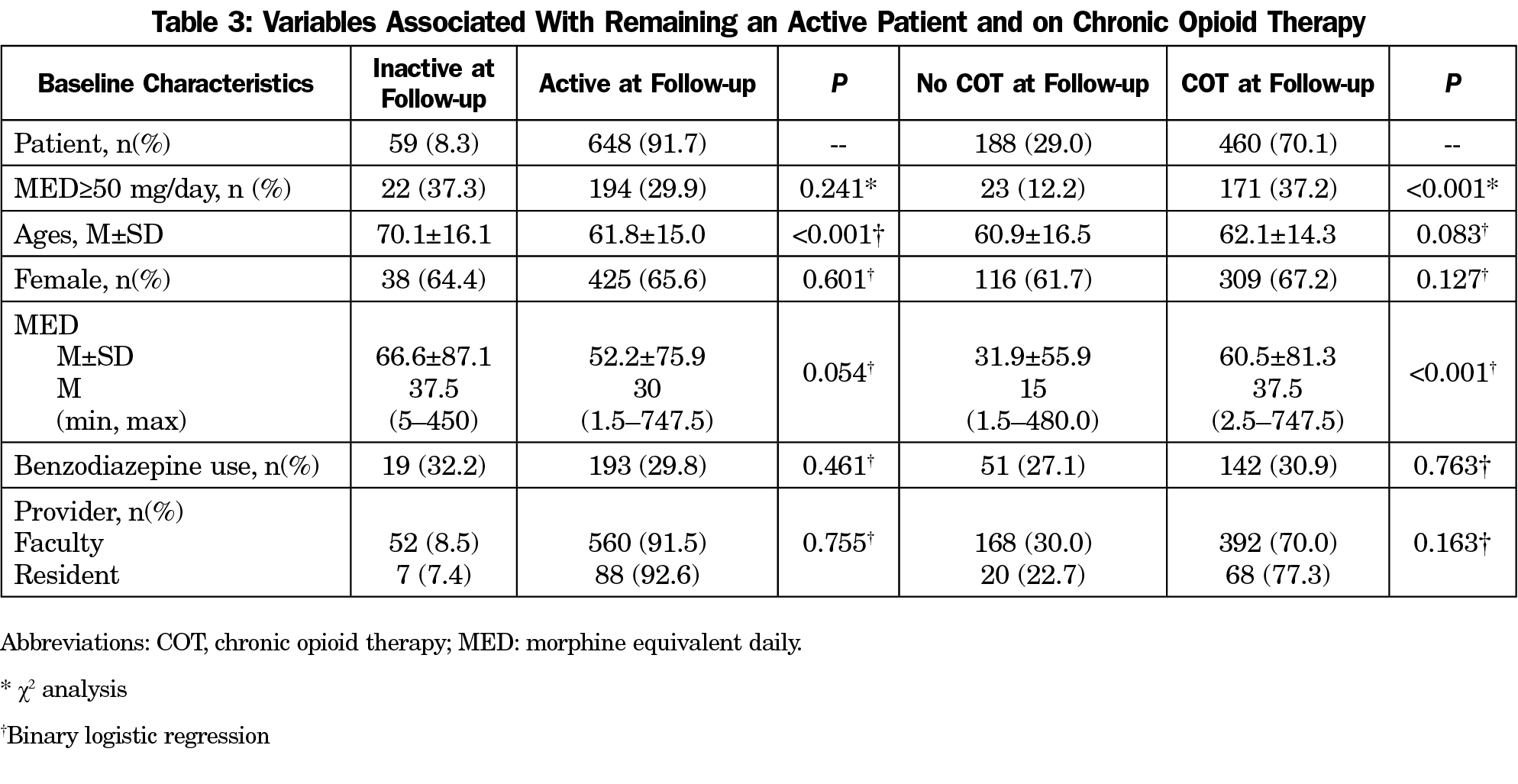
From 1 April 2019, gabapentin and pregabalin will be reclassified as Schedule 3 controlled drugs under the Misuse of Drugs Regulations 2001, and Class C of the Misuse of Drugs Act 1971. Eight states have made gabapentin a schedule V controlled substance. And 12 other states require stricter reporting on gabapentin prescriptions. If you have a prescription for gabapentin, it’s best to take the lowest dose possible. Instalments and repeatable prescriptions. Prescriptions for Schedule 2 or 3 Controlled Drugs can be dispensed by instalments. An instalment prescription must have an instalment direction including both the dose and the instalment amount specified separately on the prescription, and it must also state the interval between each time the medicine can be supplied. The Home Office has recently reclassified gabapentin and pregabalin as Schedule 3 controlled drugs. In order to maintain appropriate access for those patients who do obtain substantial pain relief and to minimise misuse and abuse there is a need for a comprehensive understanding of the safety and harms of gabapentinoids. services and key stakeholders about the expectations for the handling of gabapentin and pregabalin as Schedule (Sch) 3 Controlled Drugs (CDs) from 1 April 2019. Background In January 2016, the Advisory Committee for the Misuse of Drugs recommended that pregabalin and gabapentin are scheduled as Sch 3 CDs within the Misuse of Drugs MHRA/CHM advice: Gabapentin (Neurontin®) and risk of abuse and dependence: new scheduling requirements from 1 April (April 2019) Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Gabapentin is approved to treat postherpetic neuralgia and epilepsy with partial-onset seizures. The large majority of gabapentin prescribing is off label. Gabapentin may be abused for euphoria, potentiating the high from opiates, reduction of alcohol cravings, a cocaine-like high, as well as sedation or sleep. Individuals at the highest risk for abusing gabapentin include those with opioid Here we explain the prescribing and dispensing changes that will need to take place when pregabalin and gabapentin become Schedule 3 Controlled Drugs. Gabapentin and pregabalin are in schedule 3, but not in the “must be kept locked in a CD cabinet” schedule 3 list. Hence, they do not need to be kept in the CD cabinet, recorded in the CD register or given with a witness. Examples of Schedule 3 CDs include tramadol, gabapentin, and pregabalin. Schedule 3 CDs are exempt from safe custody requirements, however, the RCVS advises that all Schedule 3 CDs should be stored in a CD cabinet. CDs in Schedule 4 are divided into two parts. Child 12–17 years Initially 300 mg once daily on day 1, then 300 mg twice daily on day 2, then 300 mg 3 times a day on day 3, alternatively initially 300 mg 3 times a day on day 1, then increased in steps of 300 mg every 2–3 days in 3 divided doses, adjusted according to response; usual dose 0.9–3.6 g daily in 3 divided doses (max. per dose 1.6 g 3 times a day), some children may not The exact mechanisms through which gabapentin exerts its analgesic and antiepileptic actions are unknown however, according to ; information on the FDA-approved label for the gabapentin, gabapentin has no effect on GABA binding, uptake or degradation. In, vitro studies have shown that gabapentin binds to auxiliary α2-δ subunits of voltage- As of 1 April 2019, pregabalin and gabapentin are classified as Class C controlled substances (under the Misuse of Drugs Act 1971) and scheduled under the Misuse of Drugs Regulations 2001 (as From midnight on 1st April 2019, gabapentin and pregabalin will be reclassified as Schedule 3 controlled drugs, under the Misuse of Drugs Regulations (2001), and Class C of the Misuse of Drugs Act (1971), as is already the case with Tramadol. Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. Gabapentin has been designated as a monitored prescription drug, not a controlled substance. A DEA registration number is not required for a practitioner to prescribe Gabapentin, nor is a DEA registration number required for a dispenser to fill a prescription for Gabapentin. Practical Impact for Many Prescribers and Dispensers of Gabapentin Gabapentin is not currently listed as a controlled substance under federal law. However, some states classify gabapentin as a Schedule V substance or a drug of concern and mandate reporting to PMP. Furthermore, other states are considering similar actions due to increasing evidence of associated risks. A. Schedule 2 and 3 CDs cannot be prescribed as part of the NHS repeat dispensing scheme. As of 1st April 2019, this means gabapentin and pregabalin will not be eligible for repeatable prescriptions. Q. Will pharmacies be remunerated for the Schedule 3 CD fee if prescriptions for gabapentin and pregabalin are From 1 April 2019, gabapentin and pregabalin will be reclassified as Schedule 3 controlled drugs under the Misuse of Drugs Regulations 2001, and Class C of the Misuse of Drugs Act 1971. While all other Schedule 3 CDs, including tramadol, pentazocine, the barbiturates, gabapentin, and pregabalin, as well as Schedule 2 drug quinalbarbitone are not subject to the same Safe Custody Regulations, it is an RCVS requirement that they are securely locked away.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 | |
 |  |
 |  |
 |  |