Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
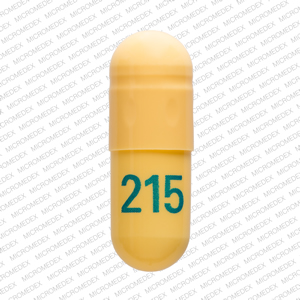
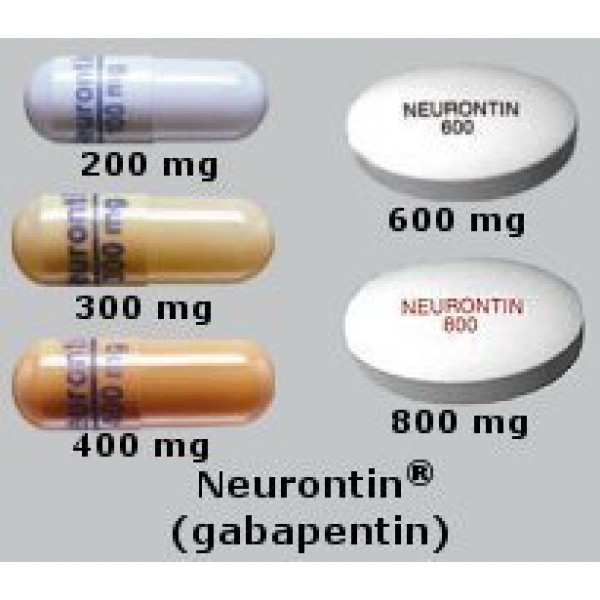
The NDC code 53451-0101 is assigned by the FDA to the product Horizant which is a human prescription drug product labeled by Azurity Pharmaceuticals, Inc. (formerly 0093-4443 : Gabapentin 600 mg Oral Tablet - Manufactured by Teva Pharmaceuticals USA Inc - Rev. Date July 11, 2008 - RxChat NDC Database. NDC Package Codes: HORIZANT Extended-Release Tablets containing 600 mg of gabapentin enacarbil are white to off-white, with occasional black/grey spots, oval-shaped tablets debossed with "GS LFG". They are supplied as follows: 300 mg: NDC 53451-0103-1: Bottles of 30 600 mg: NDC 53451-0101-1: Bottles of 30 Complete details for NDC 65862-0523-01 Gabapentin 600 mg/1 including product information, packaging information, pricing, prescribing information and package photos. GPI: 72600030000330 The NDC code 42806-657 is assigned by the FDA to the product Gabapentin which is a human prescription drug product labeled by Epic Pharma, Llc. The product's dosage form is tablet and is administered via oral form. The product is distributed in a single package with assigned NDC code 42806-657-09 90 tablet in 1 bottle . The NDC Packaged Code 68382-607-16 is assigned to a package of 90 tablet in 1 bottle of Gabapentin, a human prescription drug labeled by Zydus Pharmaceuticals Usa Inc.. The product's dosage form is tablet and is administered via oral form. Yes, Gabapentin with product code 68462-126 is active and included in the NDC Directory. The product was first marketed by Glenmark Pharmaceuticals Inc., Usa on April 01, 2006 and its listing in the NDC Directory is set to expire on December 31, 2025 if the product is not updated or renewed by the manufacturer. NDC 70771-1861-9 in bottle of 90 tablets. Gabapentin tablets, 300 mg. Rx only. 90 tablets The NDC Code 42806-657-09 is assigned to “Gabapentin ” (also known as: “Gabapentin”), a human prescription drug labeled by “Epic Pharma, LLC”. The product's dosage form is tablet, and is administered via oral form. NDC 70771-1862-9 in bottle of 90 tablets. Gabapentin tablets, 600 mg. Rx only. 90 tablets Manufacturers of Gabapentin that have been granted an NDC (National Drug Code). View NDC Code(s) NEW! NDC Code(s): 65841-705-01, 65841-705-05, 65841-705-10, 65841-706-01, view more List of products in the National Drug Code with proprietary name gabapentin. Gabapentin is used with other medications to prevent and control seizures. It is also used to relieve nerve pain following shingles (a painful rash due to herpes zoster infection) in adults. The product is distributed in 2 packages with NDC codes 16571-116-01, 16571-116-50.Gab. Search. Home; Lookup Tools. RxCUI: 310433 - gabapentin 600 MG Oral Tablet; What is NDC 45963-556? The NDC code 45963-556 is assigned by the FDA to the product Gabapentin which is a human prescription drug product labeled by Actavis Pharma, Inc.. The product's dosage form is capsule and is administered via oral form. Yes, Gabapentin with product code 50228-177 is active and included in the NDC Directory. The product was first marketed by Sciegen Pharmaceuticals, Inc. on February 04, 2016 and its listing in the NDC Directory is set to expire on December 31, 2025 if the product is not updated or renewed by the manufacturer. The product is distributed in a single package with NDC code 52427-806-90.This medication is used . Search. Home; 1115013 - Once-Daily gabapentin 600 MG Oral Complete details for NDC 68462-0126-05 Gabapentin 600 mg/1 including product information, packaging information, pricing, prescribing information and package photos. GPI: 72600030000330. Yes, Gabapentin with product code 70010-227 is active and included in the NDC Directory. The product was first marketed by Granules Pharmaceuticals Inc. on June 27, 2023 and its listing in the NDC Directory is set to expire on December 31, 2025 if the product is not updated or renewed by the manufacturer. No, Gabapentin with product code 69097-814 is excluded from the NDC Directory because it was discontinued by the manufacturer. The product was first marketed by Cipla Usa Inc. on June 29, 2016 and its listing in the NDC Directory is set to expire on September 11, 2024 if the product is not updated or renewed by the manufacturer.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |