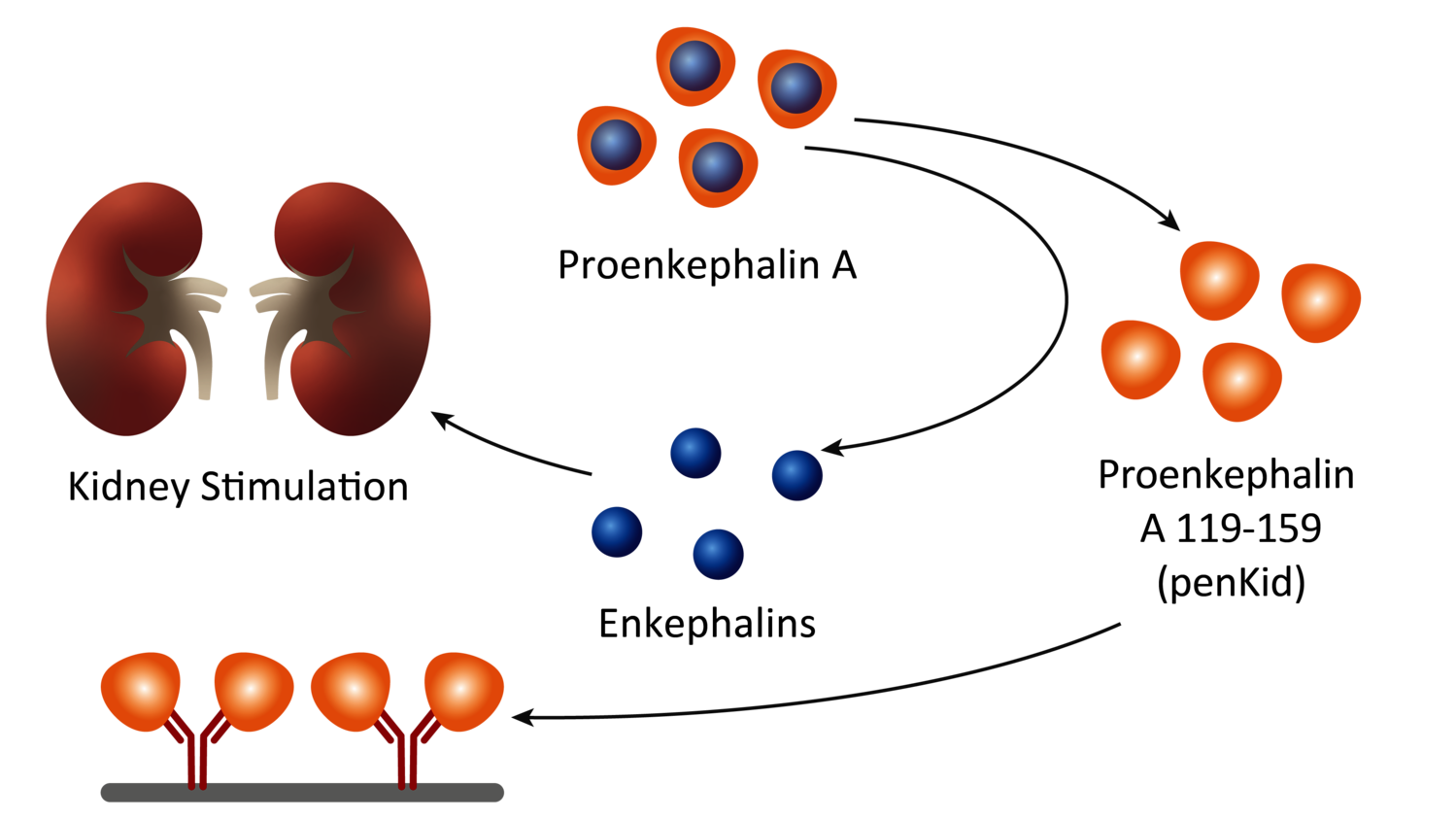
Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
Gabapentin is frequently used as an analgesic in patients with chronic kidney disease. Although gabapentin is well known for its favorable pharmacokinetics, it is exclusively eliminated renally, and patients with chronic kidney disease are at risk for toxicity. Existing literature on such risk is lacking. We found that patients with chronic kidney disease had elevated serum gabapentin concentrations, in some cases leading to gabapentin toxicity; those with advanced age and multiple comorbidities were more prone to the toxicity, and the toxicity tended to be underrecognized. Nephrotoxicity is defined as a rapid deterioration in kidney function caused by the toxic effect of drugs or chemicals. Cohort studies suggest that drug-induced nephrotoxicity could occur in as many as 14–26% of AKI episodes. Drug-induced AKI can be either dose related or idiosyncratic. We report a case of gabapentin toxicity in a patient on long-term PD who was treated with continuous automated cycling PD. We find that continuous PD provides significant clearance of gabapentin. Discussion: Gabapentin is widely used in the management of pain. It is entirely excreted through the renal system so this needs to be considered in any patient becoming acutely ill and developing renal failure. We describe a patient who developed significant deterioration in her conscious level due to iatrogenic gabapentin overdose. Nephrotoxic medications can elicit damage to the kidney via various mechanisms, including alteration in its structure and function. When evaluating the primary etiologies in renal injury, the incidence of drug-induced toxicity has accounted for 20% of all-cause incidents. Potentially nephrotoxic drugs are more likely to cause harm at higher doses, with longer durations of therapy, and/or in combination with other nephrotoxic agents. Impaired drug elimination becomes increasingly prominent as kidney function declines and is most challenging for patients on renal replacement therapy (RRT, or dialysis). Patients with chronic kidney disease often receive inappropriately high gabapentin dosage for their kidney function, occasioning overt toxicity; advanced age and comorbidity predispose these patients for toxicity. We examined the Mayo Clinic Rochester database from 1998 to 2007 in patients with serum gabapentin measurements and known medical outcomes. A total of 729 patients were stratified according to their estimated glomerular filtration rate: group I, 126 individuals with estimated glomerular filtration greater than 90 mL/min/1.72 mm 3; group II, 594 individuals with estimated glomerular filtration Certain drugs are inherently nephrotoxic and include aminoglycosides, amphotericin B, cisplatin, contrast dye, and cyclosporine. 7, 34 For others, such as those associated with chronic Due to the patient history mentioned above, we screened urine for toxins, and it was positive for cocaine, heroin and morphine. We also ran a full blood and urine analysis which provided the following relevant results: When it comes to gabapentin and kidney disease, kidney disease sufferers should be aware of the risks that are involved in taking gabapentin with kidney disease. Gabapentin is actually toxic to the kidneys. This case report outlines a significant type of morbidity due to continued use of gabapentin during an episode of acute renal failure. Setting. University teaching hospital. Discussion. Gabapentin is widely used in the management of pain. However, carambola, with its unspecified toxin, is nephrotoxic while gabapentin does not seem to affect renal function and its duration of effect depends solely on creatinine clearance. In addition, the adverse effects of gabapentin on the central nervous system vary from person to person. Gabapentin and pregabalin are fully excreted unchanged by the kidney Drugs and effects on kidney function Nephrotoxicity is defined as a rapid deterioration in kidney function caused by the toxic effect of drugs or chemicals. Drug dosing errors are common in patients with renal impairment and can cause adverse effects and poor outcomes. Dosages of drugs cleared renally should be adjusted according to creatinine Child 6–11 years 10 mg/kg once daily (max. per dose 300 mg) on day 1, then 10 mg/kg twice daily (max. per dose 300 mg) on day 2, then 10 mg/kg 3 times a day (max. per dose 300 mg) on day 3; usual dose 25–35 mg/kg daily in 3 divided doses, some children may not tolerate daily increments; longer intervals (up to weekly) may be more appropriate, daily dose maximum to be given in 3 divided Gabapentin contains a cyclohexyl group and is a form of gamma-aminobutyric acid (GABA). Despite its name, gabapentin does not affect the inhibitory neurotransmitter GABA or its receptors. Instead, it acts as a ligand, binding strongly to the α2δ Gabapentinoids are opioid substitutes whose elimination by the kidneys is reduced as kidney function declines. To inform their safe prescribing in older adults with chronic kidney disease (CKD), we examined the 30-day risk of serious adverse events according to the prescribed starting dose. Population-based cohort study. Forty patients exhibited gabapentin toxicity, 0 in group I, 5.56% (33/594) in group II, and 77.8% (7/9) in group III; their mean serum gabapentin concentrations were 29.1 2.46 g/mL and 54.7 15.9 Patients with Chronic Kidney Disease withg/mL, respectively. The low-est serum gabapentin level among the symptomatic individ-
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |