Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 | |
 |  |
 |  |
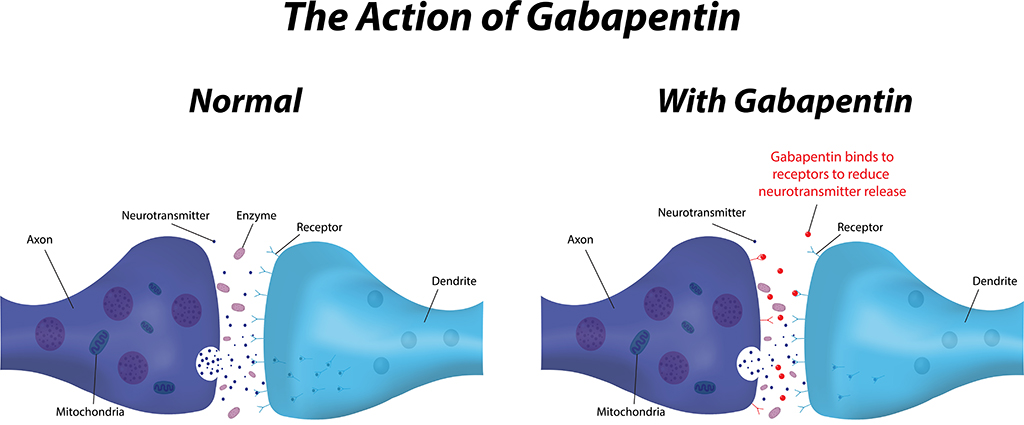
Gabapentin, also known by the brand name Neurontin, is an anticonvulsant medication primarily used to treat seizures and manage neuropathic pain. It is a synthetic medication that works by regulating certain neurotransmitters in the brain. What is Gabapentin? In 2004, Pfizer agreed to pay $430 million in a DOJ settlement and pleaded guilty to two violations of the Food, Drug and Cosmetic Act for marketing the drug Neurontin, also known as gabapentin The Pfizer Gabapentin Lawsuit: Unpacking the Controversy. The Core of the Allegations; The Settlement and Legal Consequences; Ongoing Concerns and Safety Issues; Frequently Asked Questions (FAQs) 1. What specifically did Pfizer do wrong with gabapentin marketing? 2. How much money did Pfizer pay in total for the gabapentin lawsuit? 3. Pfizer, the world's largest drug maker, pleaded guilty on 13 May to numerous civil and criminal charges for illegally promoting the off-label use of gabapentin (Neurontin). It has agreed to pay a $240m (£136m; €200m) criminal fine and $152m to state and federal healthcare programmes. The fine is the second largest given in the industry. The Neurontin Consumer Antitrust Class Action Lawsuit is In re: Neurontin Antitrust Litigation, Case No. 2:02-cv-01390, in the U.S. District Court for the District of New Jersey. UPDATE: Instructions on how to file a claim for the Neurontin third-party payor class action settlement are now available! Neurontin Lawsuit | 2025 Latest Updates The prescription pain reliever Neurontin (generic gabapentin) has recently been linked to an increased risk for Stevens-Johnson syndrome (SJS), a severe skin disorder in which the top layer of the skin dies, followed by a painful rash that spreads and blisters. The history of gabapentin includes significant lawsuits related to off-label marketing, highlighting the ethical and safety concerns of such practices. While gabapentin remains a valuable treatment option for specific conditions, awareness of its potential risks and side effects is crucial for both prescribers and patients. The information Brought by a class of third-party payers, the lawsuit alleges Pfizer and Warner-Lambert fraudently marketed Neurontin according to documents filed in Massachusetts federal court on Friday. The lawsuit against gabapentin primarily revolves around allegations that Pfizer, through its subsidiary Parke-Davis, engaged in illegal and systematic off-label marketing of the drug, Neurontin. This means that the company is accused of promoting gabapentin for uses that were not approved by the U.S. Food and Drug Administration (FDA Gabapentin lawsuits are subject to statutes of limitations, which set timeframes for filing legal claims. These deadlines typically range from one to six years for product liability and personal injury cases and usually start when the plaintiff first experiences memory issues or discovers their connection to gabapentin. Neurontin is an anticonvulsant drug used to treat partial seizures and nervous system pain. Some serious side effects have been reported. These include respiratory disease, allergic reactions, and suicidal thoughts. Potential for Gabapentin and Pregabalin Lawsuits The new warnings may lead people to file drug lawsuits over breathing-related injuries blamed on gabapentin’s and pregabalin’s respiratory risks. Poison control centers have reported increased calls about the gabapentinoids. The lawsuit alleged that Pfizer had deceptively marketed Neurontin, causing financial losses to the payers. More recently, a Gabapentin lawsuit in 2022 has been filed against Gabapentin manufacturers, accusing them of failing to warn patients about potential side effects of the medication, including memory loss. Gabapentin Lawsuit – How to Join. The gabapentin class action lawsuit was filed in 2022 against three pharmaceutical companies – Teva, Pfizer, and Greenstone – accusing them of misrepresenting the drug’s risks and overstating its benefits. To join the gabapentin lawsuit, potential class members need to meet the following criteria: WASHINGTON, D.C. – The U.S. Drug Enforcement Administration (DEA) and the U.S. Food and Drug Administration (FDA) should promptly classify the markedly overprescribed seizure and neuropathic pain drug gabapentin and the closely related drug gabapentin enacarbil as schedule V controlled substances, because they are increasingly being misused, abused, and diverted, leading to dependence and What Is Neurontin? Gabapentin, an anti-seizure drug marketed by Pfizer Laboratories in the United States under the trademark name Neurontin, has been the subject of numerous claims of malfeasance in gabapentin side effects lawsuits, with the Pfizer company intentionally and knowingly marketing the medication for uses not approved by the FDA (Food and Drug Administration). Discussion of the legal issues Neurontin (generic name: gabapentin) was FDA-approved in 1993 for use as an anticonvulsant for people suffering from partial seizures associated with epilepsy. It is also FDA-approved for the WASHINGTON – American pharmaceutical giant Pfizer Inc. and its subsidiary Pharmacia & Upjohn Company Inc. (hereinafter together "Pfizer") have agreed to pay $2.3 billion, the largest health care fraud settlement in the history of the Department of Justice, to resolve criminal and civil liability arising from the illegal promotion of certain pharmaceutical products, the Justice Department Gabapentin may cause side effects such as dizziness, drowsiness, and dizziness. It is important to follow the prescribed dosage and seek medical attention if experiencing serious side effects or changes in mood or behavior. Gabapentin is prescribed by healthcare professionals and should only be taken under medical supervision.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 | |
 |  |
 |  |