Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 | 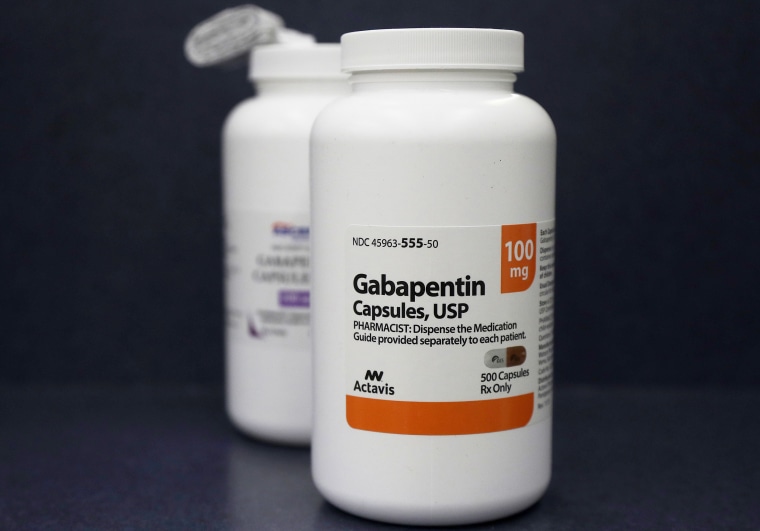 |
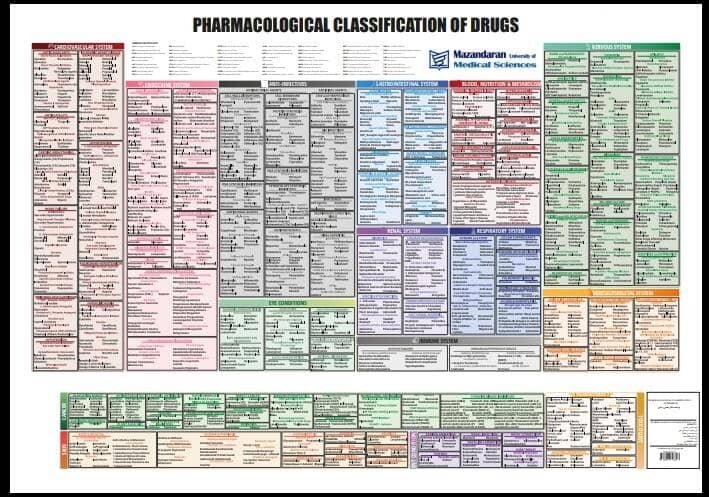
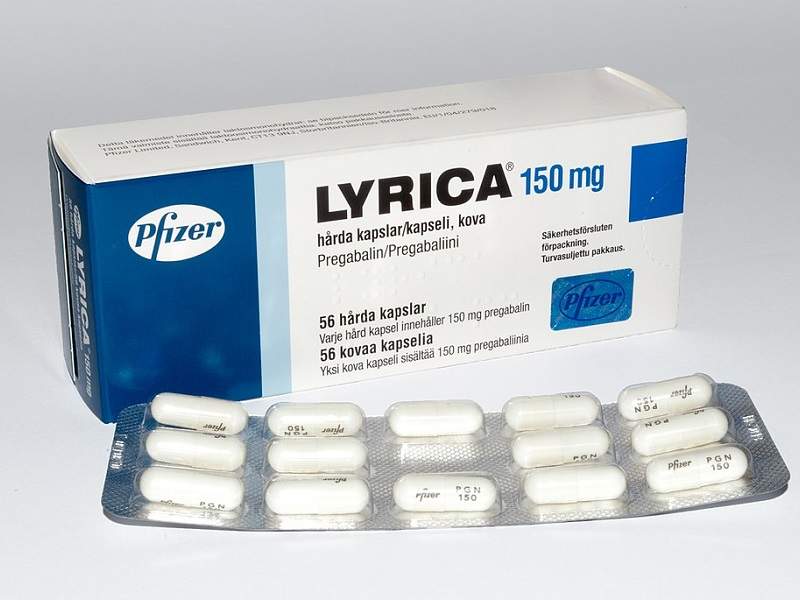
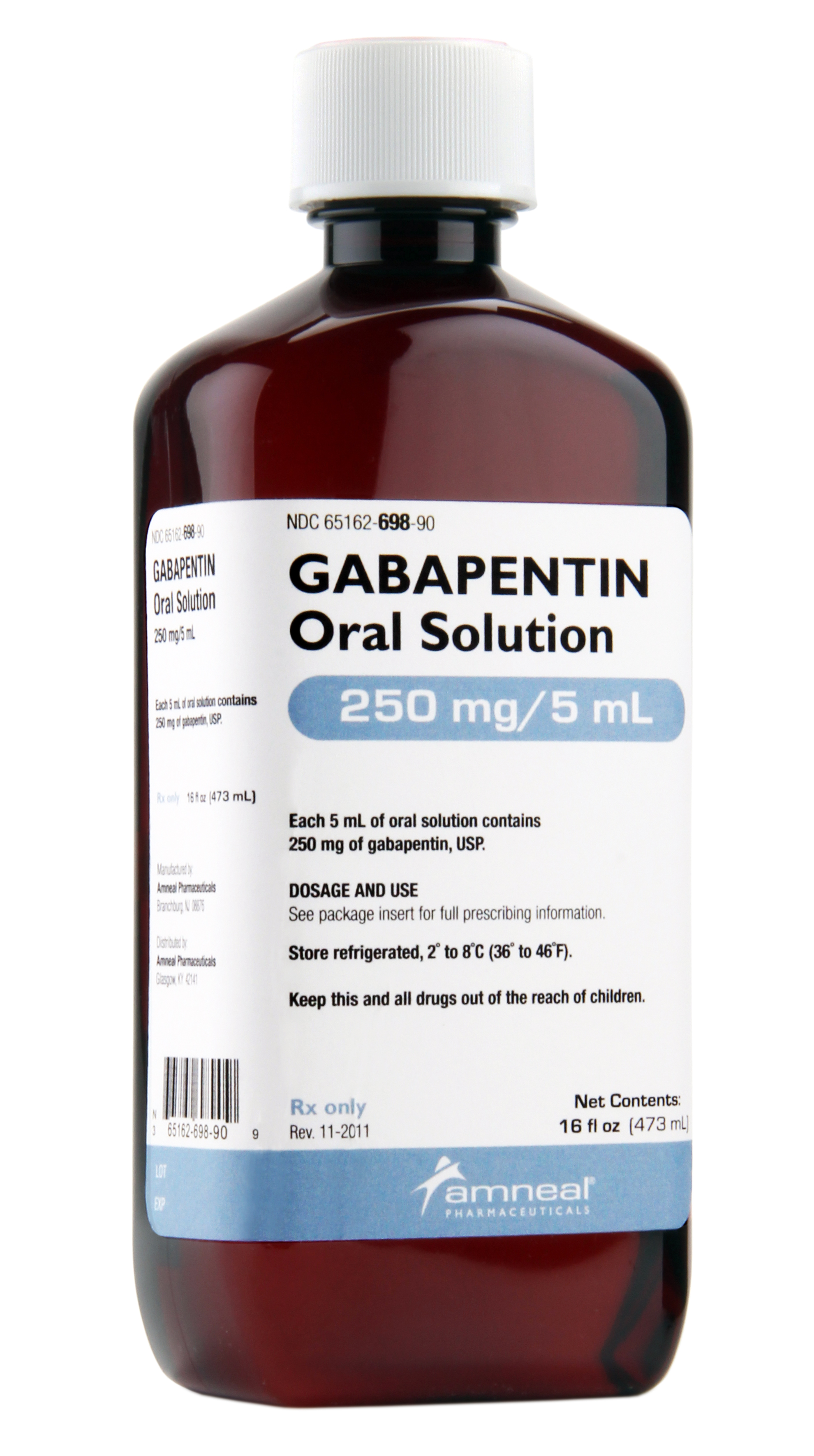
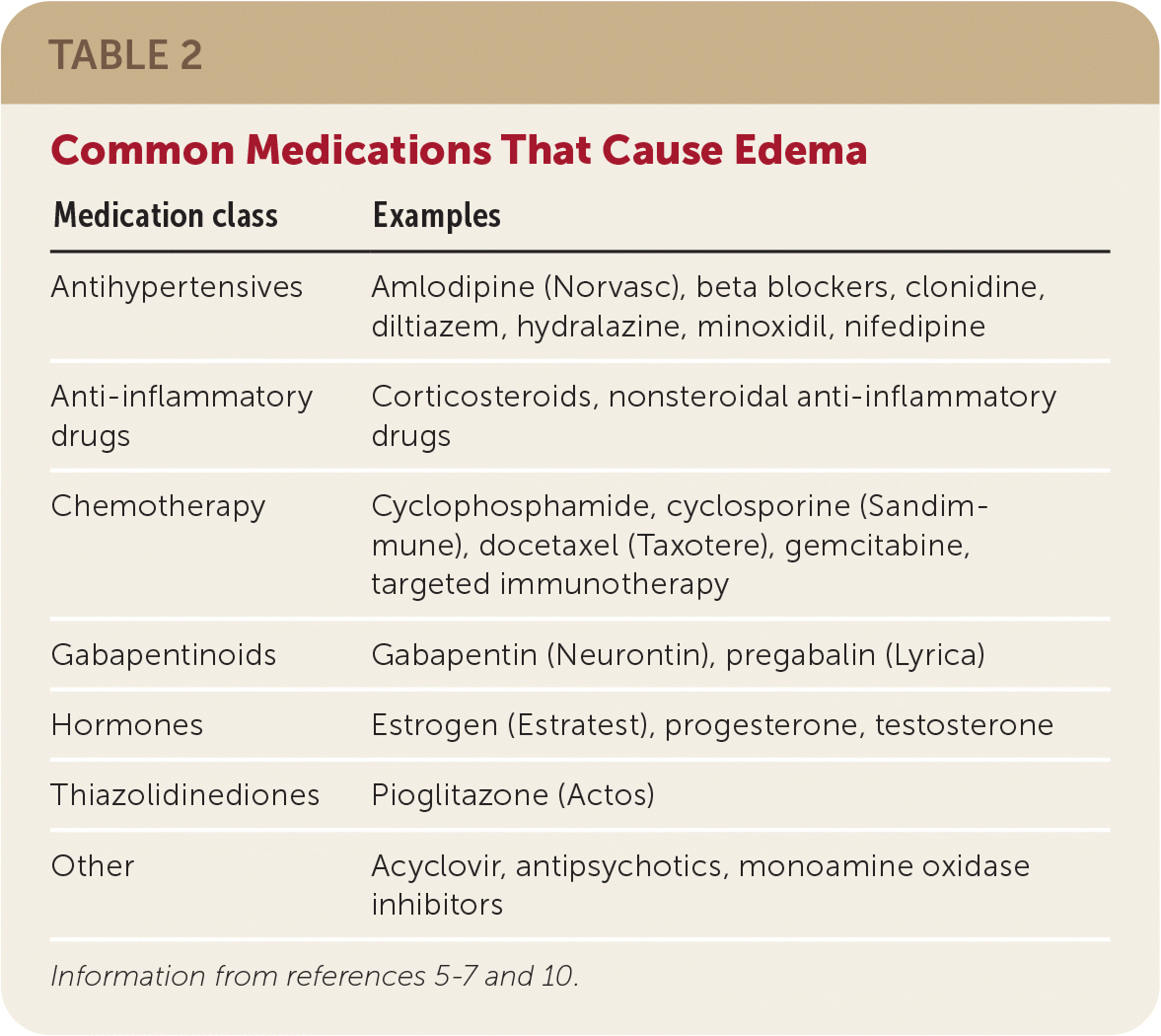
In April 2019, [59] the United Kingdom scheduled gabapentin and pregabalin as Class C drugs under the Misuse of Drugs Act 1971, and as Schedule 3 under the Misuse of Drugs Regulations 2001. [60] However, it is not a controlled substance in Canada, or Australia, and the other gabapentinoids, including phenibut, are not controlled substances Gabapentin is an anti- seizure (anti-convulsant) drug that is used for preventing seizures and for treating post-herpetic neuralgia, the pain that follows an episode of shingles. Doctors do not know how gabapentin works. Gabapentin structurally resembles the neurotransmitter gamma aminobutyric acid (GABA). Gabapentin is not the same as pregabalin, even though they both belong to the same class of medicine, called gabapentinoids, and work similarly. Lyrica and Lyrica CR are the only brands of pregabalin. Neurontin is a brand name for gabapentin. Other brands of gabapentin include Gralise and Horizant. Gabapentin (Neurontin) and pregabalin (Lyrica) both belong to a class of drugs called gabapentinoids, which means they work in similar ways. They're both used to treat chronic pain in certain Gabapentin contains the active ingredient gabapentin. Both Lyrica and gabapentin belong to a class of drugs known as anticonvulsants. The Food and Drug Administration (FDA) has approved Lyrica Following concerns about abuse, pregabalin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Healthcare professionals should evaluate patients carefully for a history of drug abuse before prescribing pregabalin, and observe patients for signs of abuse and dependence. Absorption and distribution. Pregabalin is rapidly and completely absorbed as compared to gabapentin. Peak plasma concentrations are seen within an hour as compared to 3 hours with gabapentin. 12 Oral bioavailability for pregabalin is more than 90% as compared to 30–60% for gabapentin. Gabapentin and pregabalin are members of a class of anti-convulsive and anti-epileptic drugs called gabapentinoids. Gabapentin was first approved in 1993 and pregabalin followed in 2004. They’ve been widely prescribed to treat certain types of pain as well. Conditions gabapentin and pregabalin are approved to treat include: Gabapentin is an anticonvulsant with pain-relieving effects that may be used to treat certain seizure disorders or relieve nerve pain. Common side effects include dizziness or drowsiness and it may more. Pregabalin is used in the treatment of nerve pain and also to prevent seizures. The Misuse of Drugs Act 1971 (Amendment) Order 2018 classifies pregabalin and gabapentin as Class C drugs under paragraph 1 (b) of Part 3 of Schedule 2 to the Misuse of Drugs Act 1971 (“the 1971 Prescription drugs pregabalin and gabapentin are to be reclassified as class C controlled substances from next April, the government announced today (15 October). The law change means that it will be illegal to possess Gabapentin and pregabalin without a prescription and to supply or sell it to others. What are gabapentin and pregabalin? Gabapentin and pregabalin are drugs used to treat pain. They come in multiple forms including tablet, oral solution and capsules. Find out more about managing pain in MS Pregabalin (Lyrica) and gabapentin (Neurontin) are both approved to treat nerve pain. How are they different, and which one is preferred? Compare both meds here. As of 1 April 2019, pregabalin and gabapentin are controlled under the Misuse of Drugs Act 1971 as Class C substances and scheduled under the Misuse of Drugs Regulations 2001 as Schedule 3. From 1 April 2019 pregabalin and gabapentin will be reclassified as class C controlled substances in the UK. The change, announced in October 2018, is expected to prompt a decline in the use of the drugs as prescribing, dispensing, and collecting them becomes more onerous for doctors, pharmacists, and patients. The reclassification will make it illegal to supply pregabalin and gabapentin Gabapentin and pregabalin are similar orally administered drugs but differ in their indications and dosages. Regarding safety, pregabalin is a controlled substance; gabapentin is not. Both drugs are used to treat certain types of nerve-related pain in adults. What are pregabalin and gabapentin? Pregabalin and gabapentin, collectively gabapentinoids, are primarily anticonvulsant drugs. Over the past decade, they have been increasingly prescribed for pain. 1 They are recommended for neuropathic pain in adults 2 3 (table 1), but are commonly used off-label for other pain disorders such as low back pain, sciatica, and migraine. 9 10 Pregabalin was one Pregabalin and gabapentin are both used to treat nerve pain, seizures, and anxiety, but pregabalin has faster absorption, higher bioavailability, and requires lower doses for similar effects compared to gabapentin. Both pregabalin and gabapentin are antiepileptic medications that bare structural resemblance to gamma-aminobutyric acid (GABA), though neither agent has activity in GABA’s neuronal systems. Gabapentin and pregabalin are antiepileptic drugs commonly used for neuropathic pain management and pain reduction in adults. Both medications are classified as antiepileptic medications, but they have differences in pharmacokinetics, safety profile, and clinical applications.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |