Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
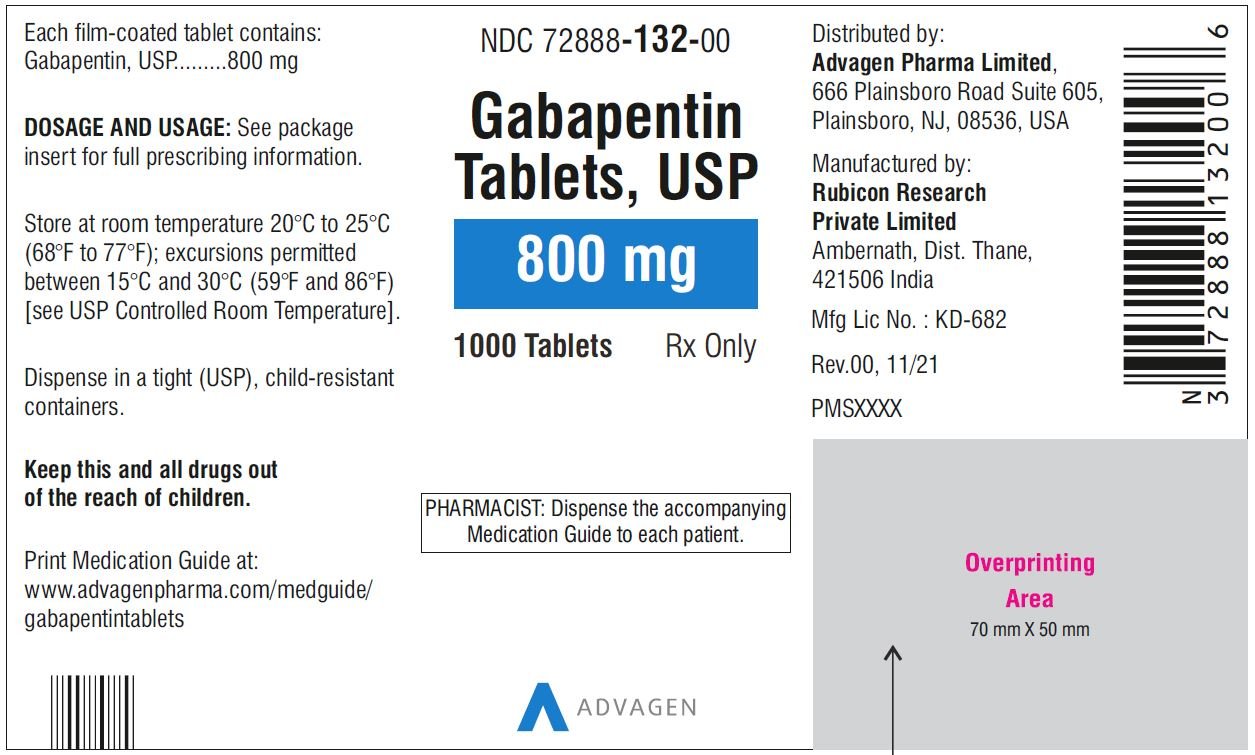
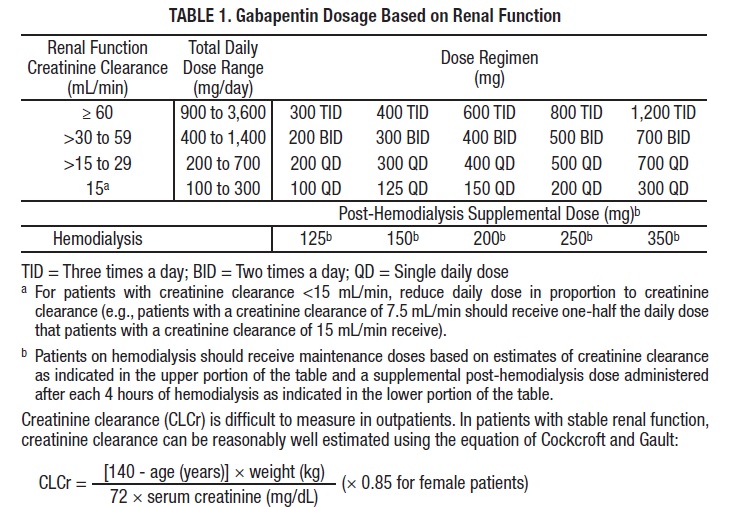
Generic (Trade Name) Dosage Form/Strength Generic Availability Gabapentin (Gralise®) Tablet: 300, 600 mg - Gabapentin (Neurontin®*) Capsule:* 100, 300, 400 mg Solution: 250 mg/5 mL Tablet:* 600, 800 mg Gabapentin enacarbil (Horizant®) Extended-release tablet: 300, 600 mg - *Generic available in at least one dosage form or strength. Gabapentin is eliminated from the systemic circulation by renal excretion as unchanged drug. Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly The starting dose range is 10 mg/kg/day to 15 mg/kg/day, given in three divided doses, and the recommended maintenance dose reached by upward titration over a period of approximately 3 days. U.S.-based MDs, DOs, NPs and PAs in full-time patient practice can register for free access to the Prescribers’ Digital Reference on PDR.net. PDR.net is to be used only as a reference aid, it is not intended to be a substitute for the exercise of professional judgement. Initial dose: The starting dose is 300 mg three times a day. Dose Range: The dose may be increased, depending on the response and tolerance of the patient, using 300 or 400 mg capsules, or 600 or 800 mg tablets 3 times a day up to 1800 mg/ day. Trusted by generations of healthcare providers, PDR.net supports prescribing decisions and patient adherence to improve health. PDR delivers critical drug information and resources trusted and relied on by prescribers for generations. Detailed Gabapentin dosage information for adults and children. Includes dosages for Restless Legs Syndrome, Epilepsy and Postherpetic Neuralgia; plus renal, liver and dialysis adjustments. The dose of gabapentin tablets should be adjusted in patients with reduced renal function, according to Table 2. Patients with reduced renal function must initiate gabapentin tablets at a daily dose of 300 mg. Gabapentin tablets should be titrated following the schedule outlined in Table 1. 3 days. The recommended maintenance dose of NEURONTIN in patients 3 to 4 years of age is 40 mg/kg/day, given in three divided doses. The recommended maintenance dose of NEURONTIN in patients 5 to 11 years of age is 25 mg/kg/day to 35 mg/kg/day, given in three divided doses. NEURONTIN may be administered as the oral solution, capsule, or tablet, or approximately 3 days. The recommended maintenance dose of gabapentin in patients 3 years to 4 years of age is 40 mg/kg/day, given in three divided doses. The recommended maintenance dose of gabapentin in patients 5 years to 11 years of age is 25 mg/kg/day to 35 mg/kg/day, given in three divided doses. Dosages up to 50 Detailed dosage guidelines and administration information for Neurontin (gabapentin). Includes dose adjustments, warnings and precautions. Pediatric Non-Opioid Analgesic Dosing Table (Starting doses < 50kg) Drug Dose Range Indication/Comments NON-OPIOID ANALGESICS Acetaminophen See acetaminophen table NSAIDS Ketorolac IM/IV: 0.5-1mg/kg (initial dose), then 0.5mg/kg q6h x 5 days max Possible renal, cardiac, GI adverse effects. Do not use in renal and hepatic impairment Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are Usual initial gabapentin dose: 300mg q8h. Usual maintenance dose: 300-600mg q8h. Maximum dosage/day: 3600 mg. [15-29]: Dosage range: 200-700mg/day. [<15]: 100-300 mg/day. Use lower end of this range for CRCL <7.5 ml/min. TABLE 1. Gabapentin Dosage Based on Renal Function. TID = Three times a day; BID = Two times a day; QD = Single daily dose. a. Gabapentin also improves sleep quality during recovery from alcohol use. The dose range was 300–3600 mg/day, with most settling in around 900 mg/day. Gabapentin may also be useful for alcohol withdrawal, but with a caveat. An increase in gabapentin AUC values have been reported when administered with hydrocodone. (7.6) An increase in gabapentin AUC values have been reported when administered with morphine. (7.7) An antacid containing aluminum hydroxide and magnesium hydroxide reduced the bioavailability of gabapentin immediate releaseby about A Cochrane review reported that 3 to 4 patients out of every 10 with either of these conditions experienced at least a 50% reduction in pain intensity when prescribed gabapentin at dosages of 1800mg-3600 mg/day (gabapentin encarbil: 1200mg-3600 mg/day). This compared with only 1 or 2 out of every 10 given a placebo (an inactive treatment). U.S.-based MDs, DOs, NPs and PAs in full-time patient practice can register for free access to the Prescribers’ Digital Reference on PDR.net. PDR.net is to be used only as a reference aid, it is not intended to be a substitute for the exercise of professional judgement. Because gabapentin is not metabolized, studies have not been conducted in patients with hepatic impairment. Renal Impairment. Dosage adjustment of GRALISE is necessary in patients with impaired renal function. GRALISE should not be administered in patients with creatinine clearance <30 mL/min or in patients undergoing hemodialysis. Usual Adult Dose for Postherpetic Neuralgia:-Initial dose: 300 mg orally on day one, 300 mg orally 2 times day on day two, then 300 mg orally 3 times a day on day three-Titrate up as needed for pain relief-Maximum dose: 1800 mg per day (600 mg orally 3 times a day) Gabapentin available under the trade name Gralise:
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |