Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |
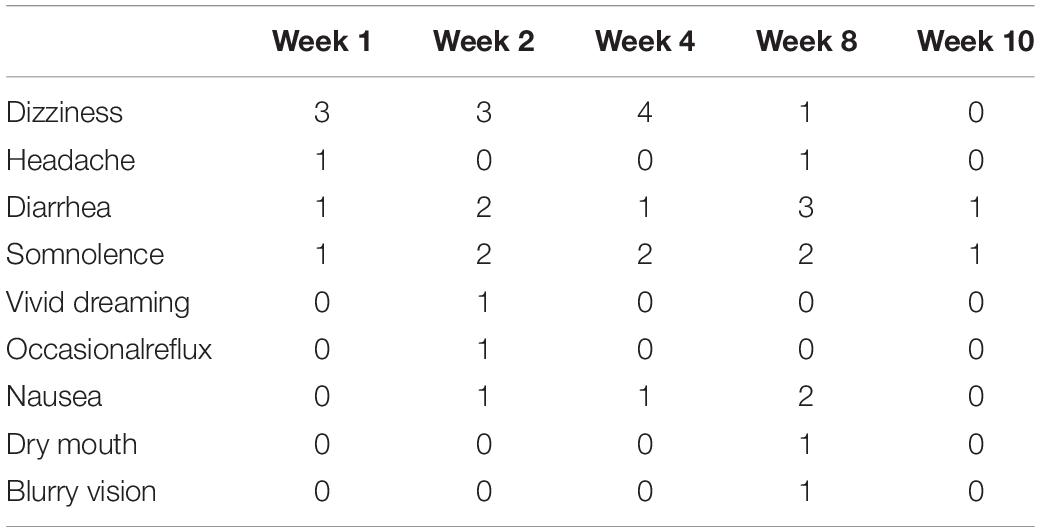
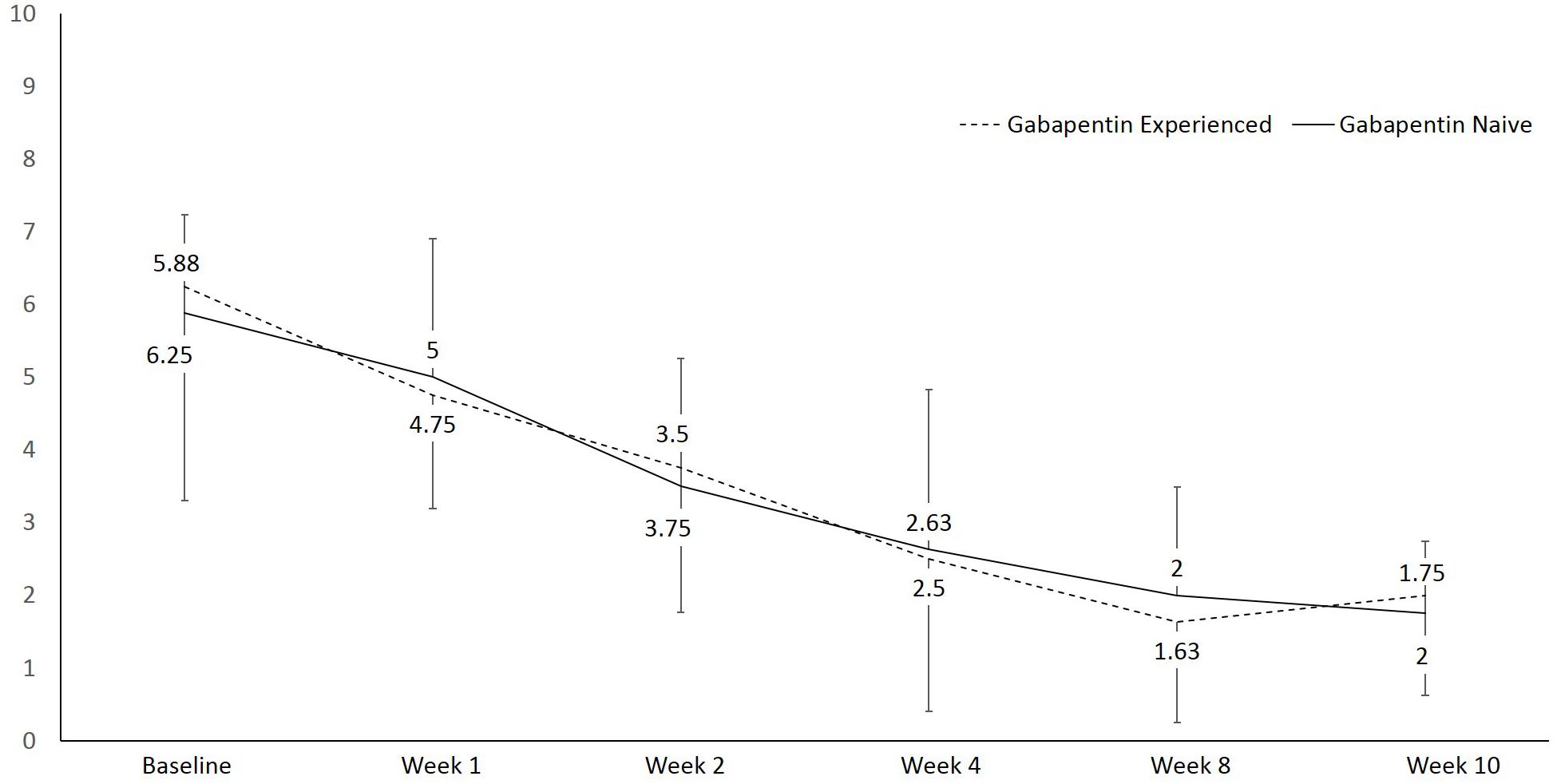
An average percent of pain relief with gabapentin extended-release was noted to be significant (p < 0.01) after 8 weeks of therapy among gabapentin-experienced (81.25 ± 16.42%) and gabapentin-naïve groups (85 ± 17.73%) when compared to baseline for gabapentin-experienced (31.25 ± 29%) and gabapentin-naïve groups (36.25 ± 34.2% Immediate release: Infants 1 month to Children 12 years: 2 to 3 hours; Adults: 2 to 4 hours; Extended release: 8 hours. Half-Life Elimination. Infants 1 month to Children 12 years: 4.7 hours. Adults, normal: 5 to 7 hours; increased half-life with decreased renal function; anuric adult patients: 132 hours; adults during hemodialysis: 3.8 hours The half-life of gabapentin in an extended release version will be significantly longer than immediate-release. The half-life of gabapentin can vary somewhat, but if someone is prescribed to take, their physician will consider this when outlining dosage instructions. GABAPENTIN (GA ba pen tin) treats nerve pain. It works by calming overactive nerves in your body. An average percent of pain relief with gabapentin extended-release was noted to be significant (p < 0.01) after 8 weeks of therapy among gabapentin-experienced (81.25 ± 16.42%) and gabapentin-naïve groups (85 ± 17.73%) when compared to baseline for gabapentin-experienced (31.25 ± 29%) and gabapentin-naïve groups (36.25 ± 34.2% Half-Life b : Urine Detection Window b: Notes: Ethanol (typically measured as ethyl glucuronide [EtG] and ethyl sulfate [EtS]) — 1-4 days: Metabolized to ethyl glucuronide and ethyl sulfate: Gabapentin (Fanatrex, Gabarone, Gralise, Horizant, Neurontin, Nupentin) 5-9 hrs: 1-2 days — Phencyclidine (1-phenylcyclohexylpiperidine, PCP, Semyl Gabapentin enacarbil is the prodrug of gabapentin; bioavailability following gabapentin enacarbil is increased in comparison to gabapentin (Backonja 2011). Current guidelines note there is insufficient evidence to recommend gabapentin encarbil in pregnant women for the treatment of restless leg syndrome (Picchietti 2015). Gabapentin is not appreciably metabolized in humans. Gabapentin elimination half-life is 5 to 7 hours and is unaltered by dose or following multiple dosing. Gabapentin elimination rate constant, plasma clearance, and renal clearance are directly proportional to creatinine clearance. Another variable that affects the gabapentin half life is the type of formula. Immediate-release gabapentin has a half life of 5 to 7 hours, but extended-release formulas are designed to release the drug more slowly, extending its action over a longer period and potentially increasing its half life. How Long Does Gabapentin Stay in Your System? *The dose is normalized to 1,000 mg equivalent (mg-eq) of gabapentin. t max is presented as median (minimum, maximum). b.i.d. = twice daily; q.d. = once daily; t.i.d. = 3 times daily; C max = maximum plasma concentration over the last dosing day; t max = time to reach maximum concentration over the dosing interval in which the C max was observed (if the maximum value occurred more than once in Extended-release (ER) drugs and immediate-release (IR) drugs with a long half-life (t 1/2) that permit once-daily dosing (such as, perampanel, zonisamide, lamotrigine [IR, ER] and topiramate [ER]) have a number of advantages over short t 1/2 ASMs that require multiple daily dosing. These advantages include simplification of dosing regimens In a study in anuric adult subjects (N=11), the apparent elimination half-life of gabapentin on nondialysis days was about 132 hours; during dialysis the apparent half-life of gabapentin was reduced to 3.8 hours. Hemodialysis thus has a significant effect on gabapentin elimination in anuric subjects. (gabapentin enacarbil) Extended-Release Tablets are indicated for the treatment of moderate-to-severe primary Restless Legs Syndrome (RLS) in adults. HORIZANT is not recommended for patients who are required to sleep during the daytime and remain awake at night. 1.2 . Management of Postherpetic Neuralgia Gabapentin enacarbil (Horizant™) extended release tablets received FDA approval in April 2011 for the treatment of moderate-to-severe primary Restless Legs Syndrome in adults. 1 Gabapentin immediate release (Neurontin) was first approved by the FDA in 1994 for the adjunct treatment of partial seizures and is also FDA approved for the In patients with Cl cr <30 mL/minute, half-life of 52 hours reported with conventional (immediate-release) gabapentin; in anuric patients, half-life reported to be 132 hours on nondialysis days and 3.8 hours during hemodialysis. The elimination half-life (t 1/2) of gabapentin ranges from 5.1 to 6.0 hours and is unaltered by dose or following multiple doses of HORIZANT. Specific Populations Race In adult patients, the half-life of gabapentin is about 5 to 7 hours. In other words, it takes the body about 5 to 7 hours to eliminate its gabapentin concentration by half. This estimate can be altered by many factors including but not limited to kidney function. Half-life for pediatric patients is roughly 4.7 hours. Under the brand Horizant, the extended-release version of gabapentin for postherpetic neuralgia, the recommended dosage is 600 mg orally twice a day. Therapy begins at 600 mg orally in the morning for three days and increases to 600 mg twice daily on day four. Gabapentin, a GABA receptor agonist, was first studied as an antiepileptic drug in humans in 1987 (07). It was launched in the United Kingdom in 1993 and approved in the United States as add-on therapy for intractable partial seizures in adults. It is also approved for the treatment of postherpetic neuralgia. Gabapentin enhances slow-wave sleep in people with primary insomnia. It also improves sleep quality by elevating sleep efficiency and decreasing spontaneous arousal.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |