Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
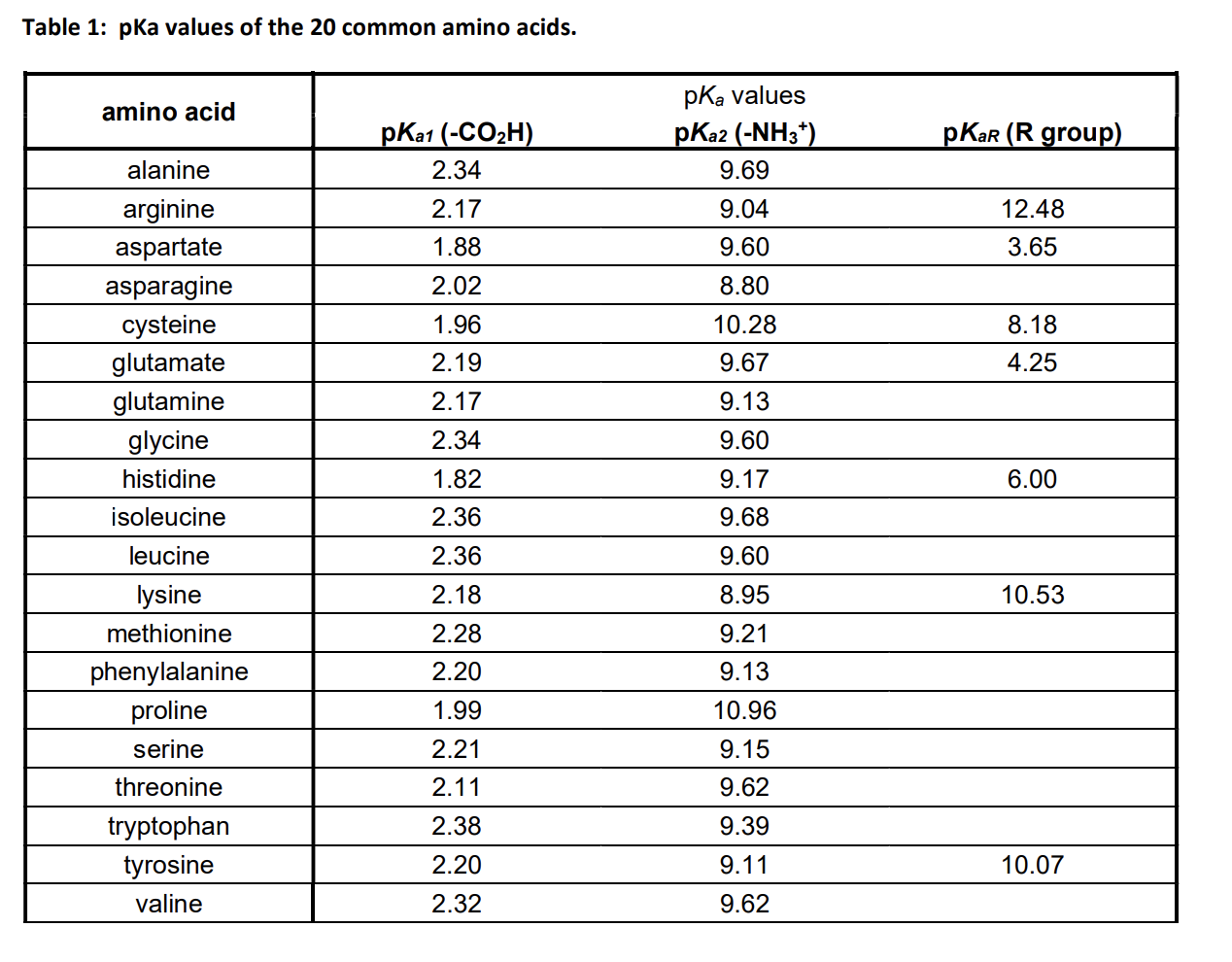 |  |
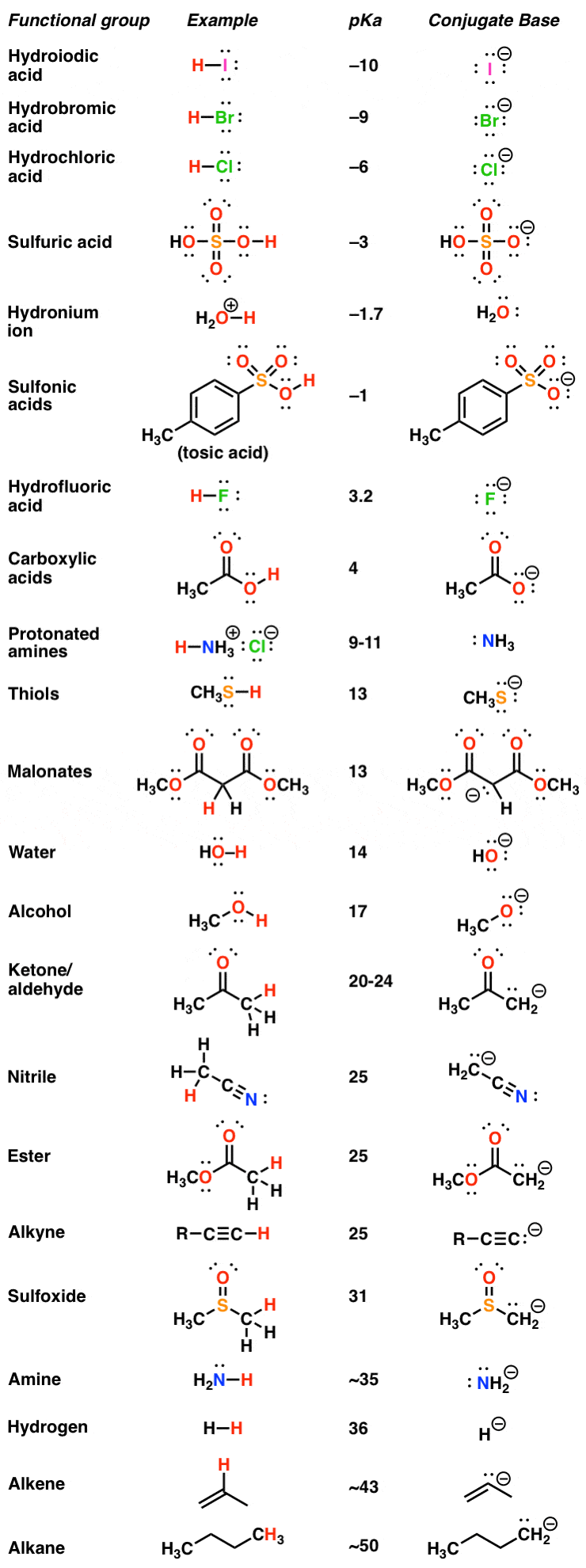
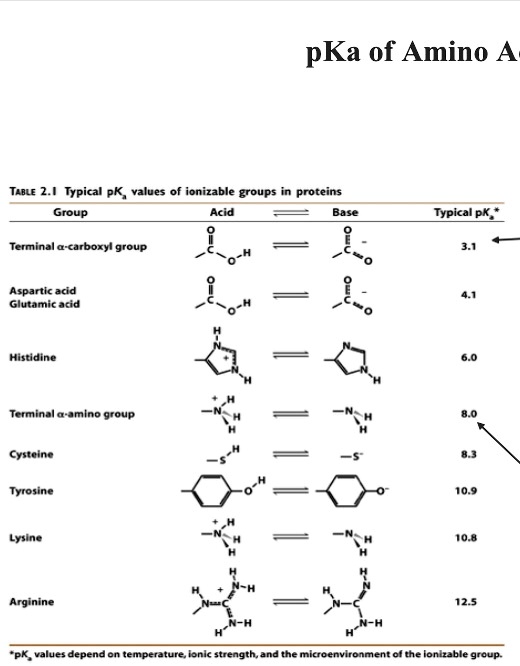
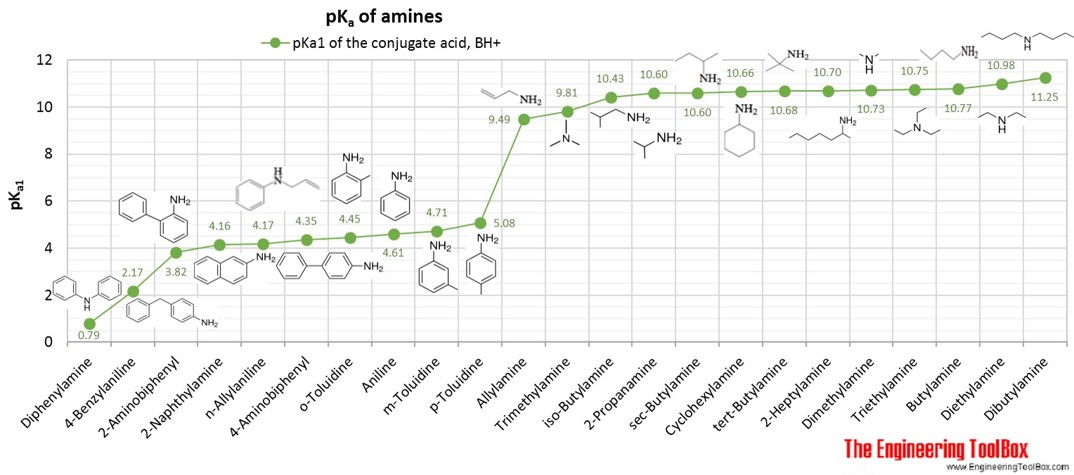
Gabapentin (l-[aminomethyl]-cyclohexaneacetic acid) is a new antiepileptic drug related in structure to GABA (Figure). It is fully water soluble, has a molecular weight of 171.34, and a pKa, of 3.68 and a pKa2 of 10.70 at 25°C. Gabapentin | C9H17NO2 | CID 3446 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. pKa is around 10.7; modestly soluble in water (10%) but extremely fat soluble. Also a substrate for L-amino acid transporters in the CNS, which means that it is concentrated in the brain, by up to ten times the plasma concentration Distribution: VOD=0.8L/kg; not bound to any plasma proteins Metabolism Gabapentin is a low molecular weight, polar molecule (log p = −1.1) which exists as a zwitterion at physiological pH and demonstrates two pKa values; 3.68 and 10.70, respectively, for the carboxylic acid and primary amine group . Gabapentin is highly water soluble, with an octanol/buffer (pH7.4) log P value of -1.10, and is zwitteronic at physiological pH (pK a value of 3.68 and 10.7) . Absolute bioavailability of gabapentin is dose dependent. The Gabapentin is small highly polar molecule which can exist as cation, anion and zwitterions due to its acid. pK a of 3.7 and base pKa of 10.7. Initially we tried with ion pairing agent like heptane sulphonic acid and octane sulphonic acid using buffer and organic modifer on C18 column. Gabapentin (CAS 60142-96-3) information, including chemical properties, structure, melting point, boiling point, density, formula, molecular weight, uses, prices, suppliers, SDS and more, available at Chemicalbook. The pharmacokinetic interactions of pregabalin and gabapentin were investigated in 12 healthy subjects following concomitant single-dose administration of 100-mg pregabalin and 300-mg gabapentin and in 18 healthy subjects following concomitant multiple-dose administration It is a zwitterion at physiological pH and has 2 pKa values (3.7 and 10.7). 6 It degrades via intramolecular cyclization to γ-lactam. 7 Figure 1 Chemical structure of gabapentin. Gabapentin interacts with cortical neurons at auxillary subunits of voltage-sensitive calcium channels Dissociation constant pKa) at 25 °C (worst case from Title: Gabapentin. CAS Registry Number: 60142-96-3. CAS Name: 1- also reported as mp 165-167° (Schmidt). pKa 1 (25°) 3.68; pKa 2 10.70. Isoelectric point 7.14 Gabapentin is a low molecular weight, polar molecule (log p = −1.1) which exists as a zwitterion at physiological pH and demonstrates two pKa values; 3.68 and 10.70, respectively, for the carboxylic acid and primary amine group . Gabapentin: Description: Gabapentin was originally developed as a chemical analogue of gamma-aminobutyric acid (GABA) to reduce the spinal reflex for the treatment of spasticity and was found to have anticonvulsant activity in various seizure models. In addition, it also displays antinociceptive activity in various animal pain models. The first pKa value is 3 (for the carboxylic acid) and the second pKa value is 10 (or the amine group). 2 Gabapentin is “freely soluble” in water and both “basic and acidic” aqueous solutions. 2 However, it is “slightly soluble” in methanol and ethanol and is “insoluble” in toluene. 2 The solubility value is >25 mg/mL. 2 pKa 1 (25°) 3.68; pKa 2 10.70. Isoelectric point 7.14. Partition coefficient (octanol/buffer): 0.075 (pH 7.4). Solubility in water at pH 7.4 exceeds 10%. 1. Gabapentin is used to treat some types of seizures, neuropathic pain, and other conditions. It has pKa values of 3.68 and 10.70. Which structures represent the protonation state of gabapentin at pH values of 2, 7, and 12? According to a classification scheme(2), this estimated Koc value suggests that gabapentin is expected to have high mobility in soil. Experimental pKa values for gabapentin are 3.68 (carboxylic acid) and 10.70 (primary amine)(4), indicating that this compound will primarily Gabapentin (GBP) is a common name for 1-(aminomethyl)cyclohexaneacetic acid (C 9 H 17 NO 2, CAS Registry No. 60142-96-3), a GABA (γ-aminobutyric acid) derivative and a popular active pharmaceutical ingredient (API). It has a molecular weight of 171.34 and two pKa values of 3.68 and 10.70. Gabapentin (GBP) is a common name for 1-(aminomethyl)cyclohexaneacetic acid (C 9 H 17 NO 2, CAS Registry No. 60142-96-3), a GABA (γ-aminobutyric acid) derivative and a popular active pharmaceutical ingredient (API) [1,2]. It has a molecular weight of 171.34 and two pKa values of 3.68 and 10.70 [3,4]. Therefore, at physiological pH, GBP exists Gabapentin, USP is a white to off-white powder with a pKa1 of 3.7 and a pKa2 of 10.7. It is freely soluble in water, 0.1 N hydrochloric acid, 0.1 N sodium hydroxide and glacial acetic acid; slightly soluble in methanol, very slightly soluble in ethanol, 2-propanol; insoluble in toluene.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |