Gallery
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 |  |
 |  |
 |  |
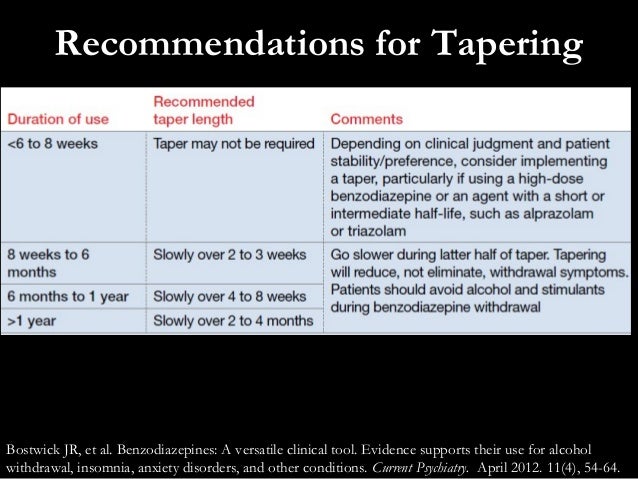
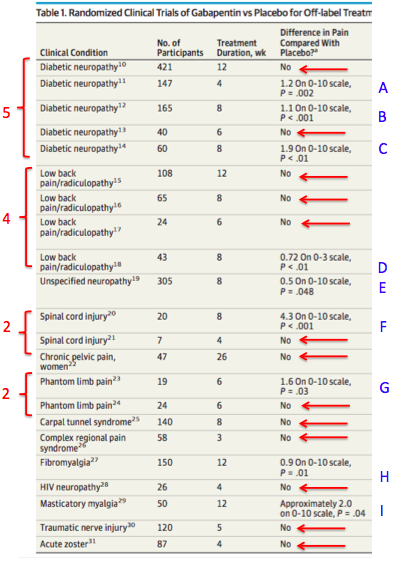
Utah law states that a prescription for a Schedule II or III opiate for an acute condition shall not be filled to exceed a seven-day supply as directed on the daily dosage rate of prescription. This limitation does tramadol should be elevated from schedule V to schedule IV; and (3) gabapentin dispensing data should be required for the controlled substances database and closely evaluated during the forthcoming year to determine if scheduling is needed during the 2020 Legislative session. 7 LONG TITLE 8 General Description: 9 This bill adds gabapentin to the list of controlled substances. 10 Highlighted Provisions: 11 This bill: 12 adds gabapentin to Schedule V of the list of controlled substances; and 13 makes technical and conforming changes. 14 Money Appropriated in this Bill: 15 None 16 Other Special Clauses: 17 None 18 Utah Code Sections Affected: 19 AMENDS: 20 58-37-4, as Pharmacies must perform an initial Gabapentin inventory on or after May 1, but before May 31, 2024. Pharmacies must include Gabapentin in their Annual Controlled Substance inventories. (1)There are established five schedules of controlled substances known as Schedules I, II, III, IV, and V which consist of substances listed in this section. (OOO)4-cyano CUMYL-BUTINACA. (W)Thebacon. (EE)1- [1- (2-thienyl)cyclohexyl]pyrrolidine, some other names: TCPy. (B)Methaqualone. Gabapentin (2-[1-(aminomethyl) cyclohexyl] acetic acid) continues in monitoring by multiple entities in the State. As described in the CSAC recommendations provided in Fall 2020 and Fall 2021, concerns about misuse of gabapentin in Utah have been expressed. This medication is approved in the Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. Utah Controlled Substances Act, 58-37 (05/08/2018) Utah Controlled Substance Act Rule, R156-37 (12/11/2017) Controlled Substance Database Act, 58-37f (05/08/2018) Controlled Substance Database Act Rule, R156-37f (12/21/2017) Utah Code Page 1 Chapter 37 Utah Controlled Substances Act 58-37-1 Short title. This act shall be known and may be cited as the "Utah Controlled Substances Act." Enacted by Chapter 145, 1971 General Session 58-37-2 Definitions. (1) As used in this chapter: (a) "Administer" means the direct application of a controlled substance, whether by because gabapentin is primarilyeliminated unchanged in the urine. Gabapentin urinary monitoring is available, and it may be used to determine if a patient is taking gabapentin or not.33 Conclusions Gabapentin is used frequently off label, and prescription numbers overall have doubled from 2011 to 2017. Gabapentin Utah's Controlled Substance Database Program (CSD) is a resource that assists prescribing practitioners and pharmacists in providing efficient care for their patients and customers usage of controlled substances. The Utah Controlled Substance Database Program was legislatively created and put into effect on July 1, 1995. Salt Lake City, Utah 84114 Telephone: (801) 538-1408; Contact a Senator; House of Representatives. 350 North State, Suite 350 PO Box 145030 Discover the current status of gabapentin scheduling as a controlled substance across the US and the PDMP requirements for each state. Valuable insights for healthcare providers. New Subsection R156-37f-203(7) requires the Utah Controlled Substance Database to track the non-controlled substance prescription drug 1 - (Aminomethyl)-cyclohexaneacetic acid (Gabapentin). New Subsection R156-37f-203(8) clarifies that the Utah Controlled Substance Database tracks derivatives of barbituric acid (Butalbital). For the 2024 legislative session the CSAC is recommending a change to the Utah CSA. Gabapentin (2-[1-(aminomethyl) cyclohexyl] acetic acid). It is the recommendation of the Controlled Substance Advisory Committee that gabapentin be scheduled as a Schedule V controlled substance in the State of Utah under Utah Code Annotated (UCA) 58-37-4(2)(e). Utah HB260 2024 General Description This bill adds gabapentin to the list of controlled substances Highlighted Provisions This bill adds gabapentin to Schedule V of After adjustment, Schedule V gabapentin regulation resulted in a reduction of 8.37 total days of gabapentin prescribed per enrollee (95% confidence interval of - 10.34 to - 6.39). In contrast, PDMP regulation resulted in a reduction of 1.01 total days of gabapentin prescribed per enrollee (95% confidence interval of - 1.74 to - 0.29). 9 This bill adds gabapentin to the list of controlled substances. 10 Highlighted Provisions: 11 This bill: 12 < adds gabapentin to Schedule V of the list of controlled substances; and 13 < makes technical and conforming changes. 14 Money Appropriated in this Bill: 15 None 16 Other Special Clauses: 17 None 18 Utah Code Sections Affected: 19 AMENDS: Eight states have made gabapentin a schedule V controlled substance. And 12 other states require stricter reporting on gabapentin prescriptions. If you have a prescription for gabapentin, it’s best to take the lowest dose possible.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 | |
 |  |
 |  |
 |  |
 |  |
 |  |