Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 | 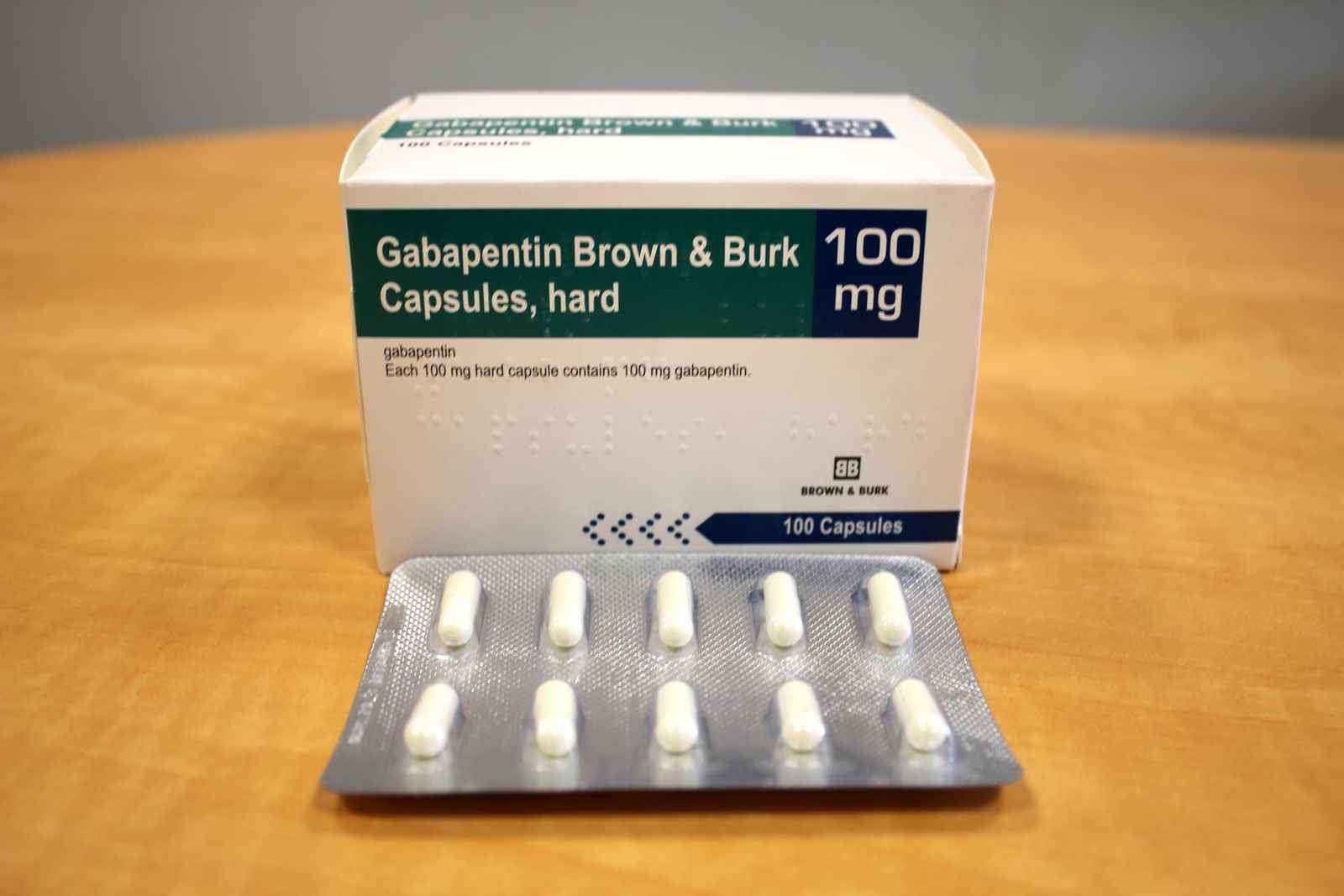 |
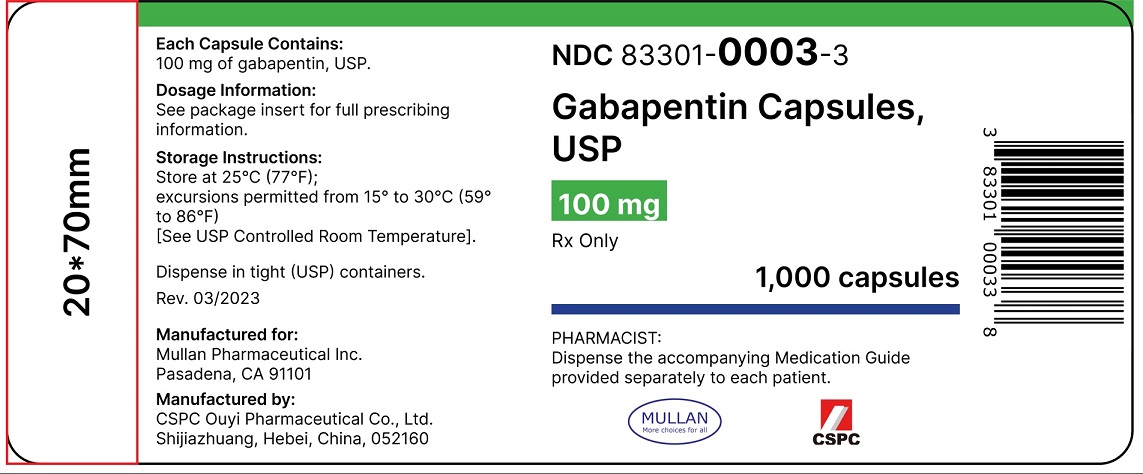
Lyrica is a medicine that contains the active substance pregabalin. It is available as capsules (white: 25, 50 and 150 mg; white and orange: 75, 225 and 300 mg; orange: 100 mg; light orange: 200 mg) and as an oral solution (20 mg/ml). Gabapentin (Neurontin and associated names) has been approved in several Member States for the treatment of epileptic syndromes and several types of neuropathic pain. The precise mechanism of action of gabapentin is not known. Gabapentin is structurally related to the neurotransmitter GABA (gammaaminobutyric acid) and interacts with GABA synapses. On 2nd September 2004, Italy (Agencizia Most frequently reported symptoms include anxiety, insomnia, nausea, pains, sweating, tremor, headache, depression, feeling abnormal, dizziness, and malaise. The occurrence of withdrawal symptoms following discontinuation of gabapentin may indicate drug dependence (see section 4.8). NEURONTIN capsules should be swallowed whole with water. Inform patients that, should they divide the scored 600 mg or 800 mg NEURONTIN tablet in order to administer a half-tablet, they should take the unused half-tablet as the next dose. Half-tablets not used within 28 days of dividing the scored tablet should be discarded. Reference ID: 4168942 Neurontin is a medication used to treat seizures and nerve pain. It works by affecting certain chemicals in the brain and nerves. It may cause drowsiness and dizziness, so caution should be taken when operating machinery or driving. Gabapentin readily enters the brain and prevents seizures in a number of animal models of epilepsy. Gabapentin does not possess affinity for either GABAA or GABAB receptor nor does it alter the metabolism of GABA. It does not bind to other neurotransmitter receptors of the brain and does not interact with sodium channels. 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. The inactive ingredients for the capsules are lactose, cornstarch, and talc. Neurontin® (gabapentin) Capsules, Neurontin (gabapentin) Tablets, and Neurontin (gabapentin) Oral Solution are supplied as imprinted hard shell capsules containing 100 mg, 300 mg, and 400 mg of Reduction of shelf life of the finished product from 36 months to 24 months for PVC/PVdC/Alu blisters pack of Gabapentin 600/800 mg Film-coated Tablet. There are product information updates. SmPC section(s) 6.3 are updated. Administer NEURONTIN three times a day using 300 mg or 400 mg capsules, or 600 mg or 800 mg tablets. The maximum time between doses should not exceed 12 hours. 3 days. The recommended maintenance Description of update: Type IB (C.I.2.a) variation application to update SmPC and PIL information in-line with the product information of reference product (Neurontin Hard capsule; EU reference No: DE/H/0899/001; MAH: Upjohn EESV) for Gabapentin Accord 100/300/400 mg hard capsules. Use gabapentin with cautions as drug reaction with eosinophilla and systemic symptoms (DRESS), a multiogram hypersensitivity with fever, rash or lymphadenopathy has occurred. Gabapentin can cause anaphylaxis and angioedema after first dose or at any time during treatment. Gabapentin is present in the breast milk of breast-feeding women. Biotransformation . There is no evidence of gabapentin metabolism in humans. Gabapentin does not induce hepatic mixed function oxidase enzymes responsible for drug metabolism. Elimination . Gabapentin is eliminated unchanged solely by renal excretion. Gabapentin is indicated as adjunctive therapy in the treatment of partial seizures with and without secondary generalization in adults and children aged 6 years and above (see section 5.1). Gabapentin is indicated as monotherapy in the treatment of partial seizures with and without Gabapentin is indicated as adjunctive therapy in the treatment of partial seizures with and without secondary generalization in adults and children aged 6 years and above (see section 5.1). 300 mg, and 400 mg of gabapentin, elliptical film-coated tablets containing 600 mg and 800 mg of gabapentin or an oral solution containing 250 mg/5 mL of gabapentin. The inactive ingredients for the capsules are lactose, cornstarch, and talc. Gabapentin is present in the breast milk of breast-feeding women. Biotransformation . There is no evidence of gabapentin metabolism in humans. Gabapentin does not induce hepatic mixed function oxidase enzymes responsible for drug metabolism. Elimination . Gabapentin is eliminated unchanged solely by renal excretion. Gabapentin readily enters the brain and prevents seizures in a number of animal models of epilepsy. Gabapentin does not possess affinity for either GABAA or GABAB receptor nor does it alter the metabolism of GABA. It does not bind to other neurotransmitter receptors of the brain and does not interact with sodium channels. Neurontin was evaluated for the management of postherpetic neuralgia (PHN) in 2 randomized, double-blind, placebo-controlled, multicenter studies; N=563 patients in the intent-to-treat (ITT) Gabapentin may have minor or moderate influence on the ability to drive and use machines. Gabapentin acts on the central nervous system and may cause drowsiness, dizziness or other related symptoms. Even, if they were only of mild or moderate degree, these undesirable effects could be potentially dangerous in patients driving or operating
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |