Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 | 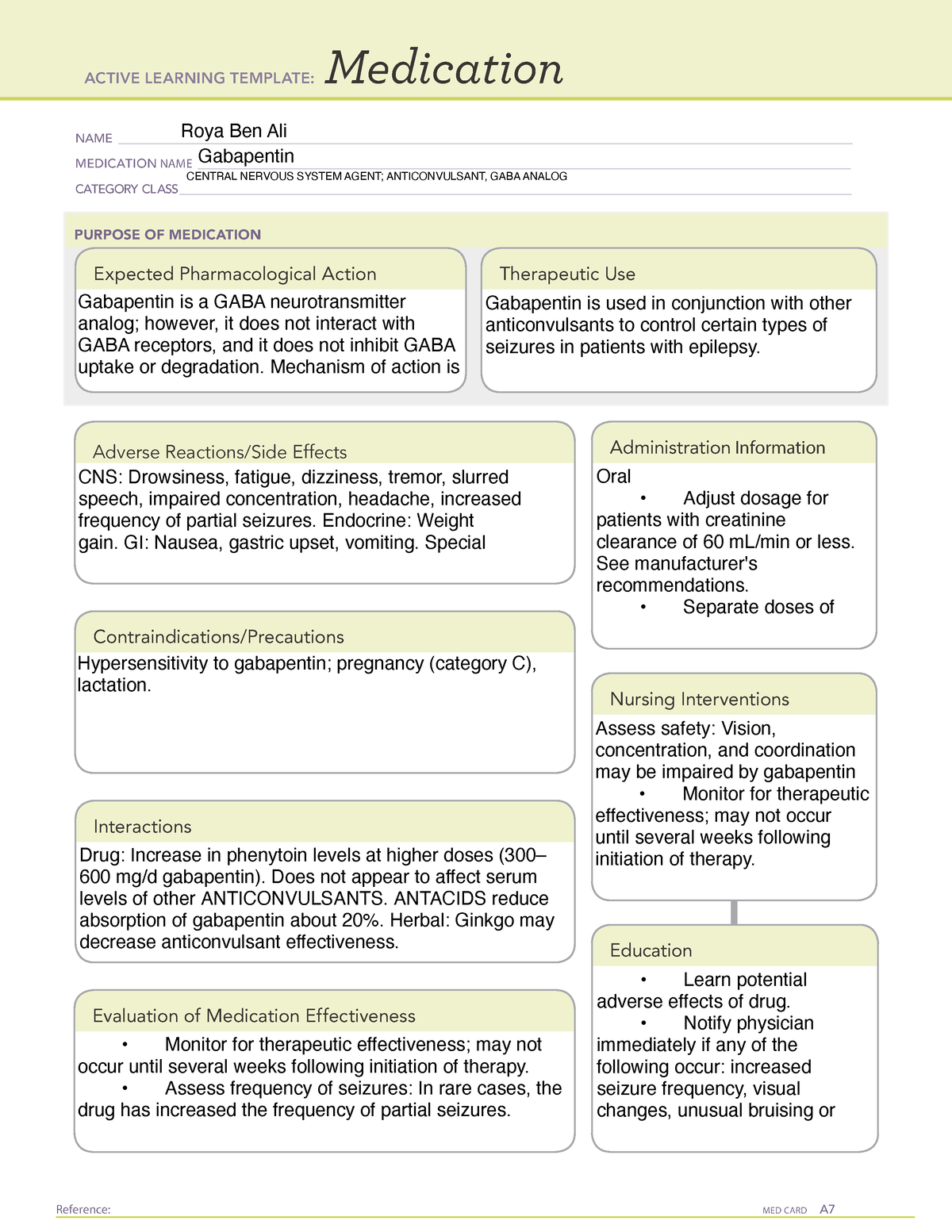 |
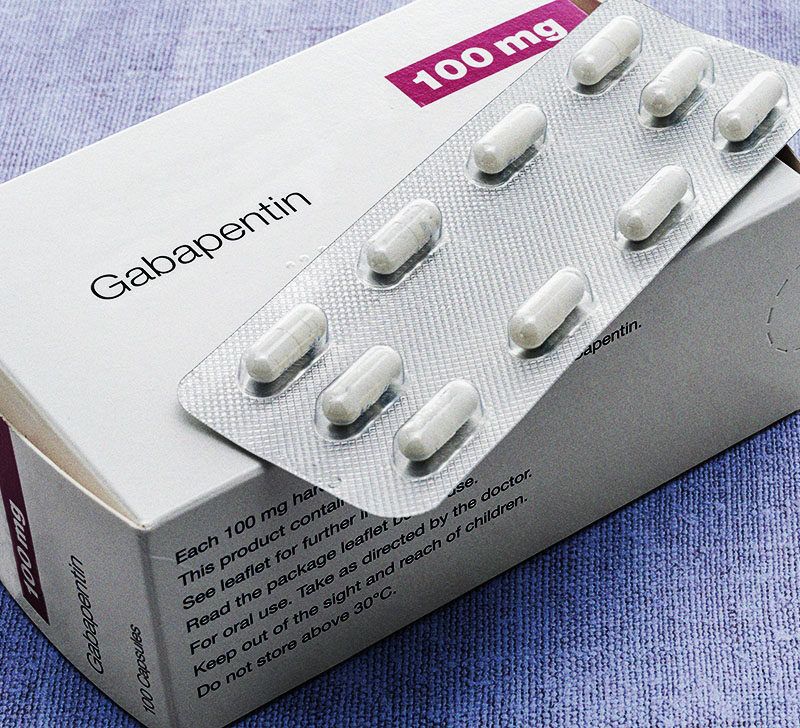
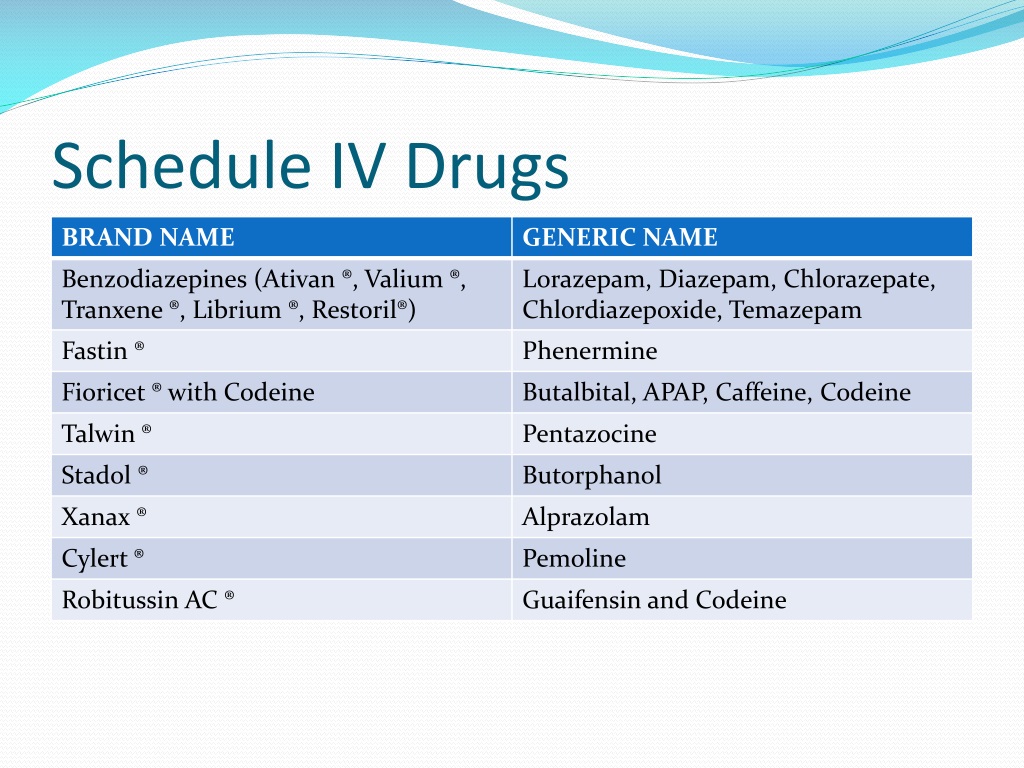
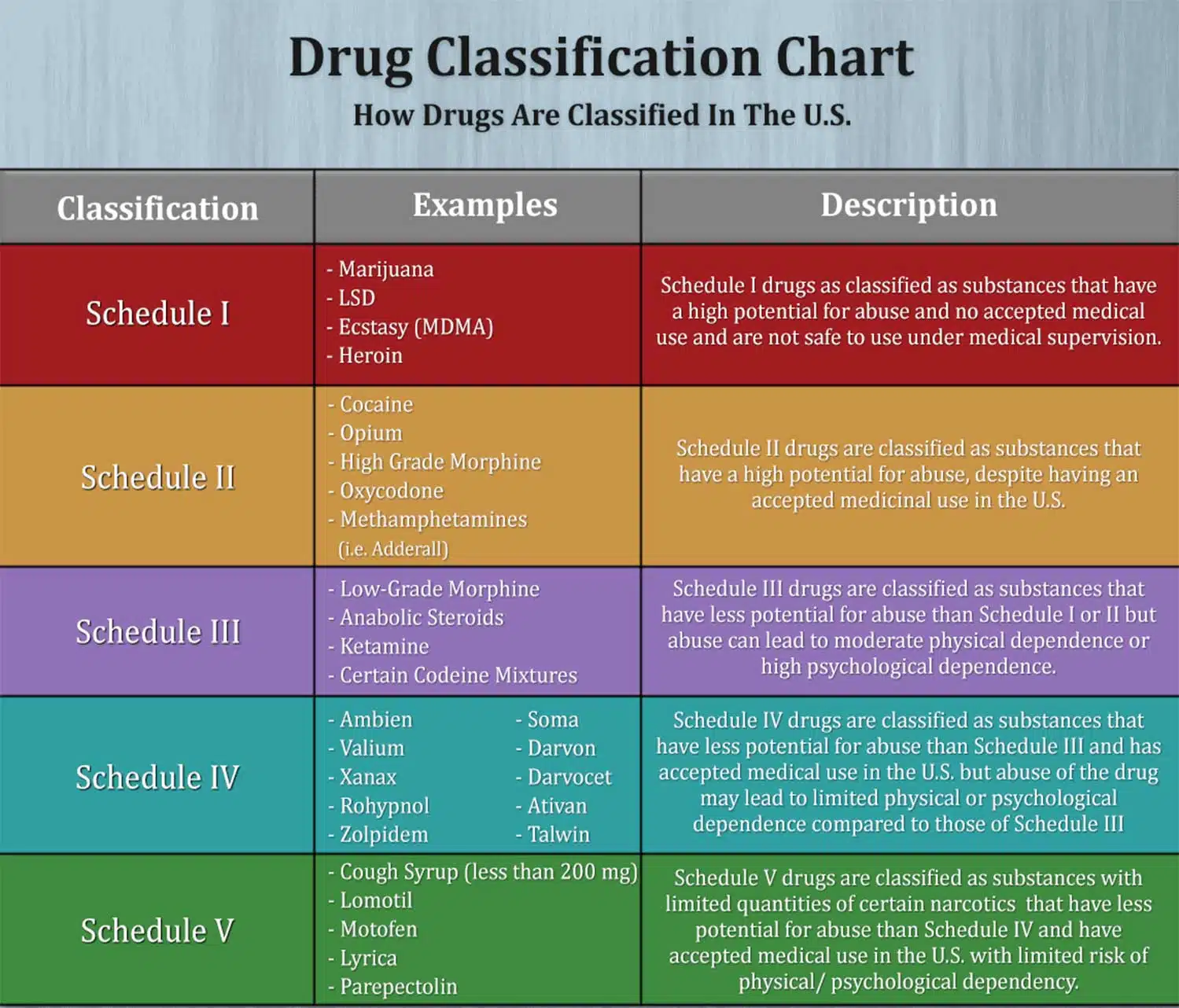
Gabapentin (Neurontin) is not a narcotic or federally controlled substance by the DEA as of November 2022, but it is classified as a Schedule V controlled substance in certain states. While all other Schedule 3 CDs, including tramadol, pentazocine, the barbiturates, gabapentin, and pregabalin, as well as Schedule 2 drug quinalbarbitone are not subject to the same Safe Custody Regulations, it is an RCVS requirement that they are securely locked away. Informstion on schedule 4 appendix b drugs. Appendix B of the Poisons and Therapeutic Goods Regulation 2008 (Regulation) lists Schedule 4 substances (prescription-only medicines) that have more stringent controls on possession and supply because they are liable to abuse, misuse and diversion. These substances are referred to und Schedule III in KY (902 KAR 55:015). Schedule IV federally. Phenobarbital & noncontrolled active ingredient; 2285 III; N Quadrapax, Phenohydro, PB Hyos elixir; Schedule III in KY (schedule IV federally). Some products with no significant potential for abuse ; specifically exempted (see 902 KAR 55:045/CFR 1308.32). Link to current list Gabapentin isn’t considered a controlled substance by the federal government as of July 2022. But several states consider gabapentin a schedule V (schedule 5) controlled substance. In states where gabapentin is a controlled substance, there’s stricter laws regarding prescribing and dispensing it from pharmacies. Gabapentin is not currently listed as a controlled substance under federal law. However, some states classify gabapentin as a Schedule V substance or a drug of concern and mandate reporting to PMP. Furthermore, other states are considering similar actions due to increasing evidence of associated risks. Gabapentin has been designated as a monitored prescription drug, not a controlled substance. A DEA registration number is not required for a practitioner to prescribe Gabapentin, nor is a DEA registration Schedules I, II, III, IV, and V shall, unless and until added pursuant to R.S. 40:962, consist of the following drugs or other substances, by whatever official name, common or usual name, chemical name, or brand name designated: SCHEDULE I A. Opiates. Unless specifically excepted or unless listed in another schedule, any of the following Appendix D of the Poisons and Therapeutic Goods Regulation 2008 (Regulation) lists Schedule 4 substances (prescription-only medicines) that have common therapeutic uses, but are also liable to abuse, misuse and diversion, warranting more stringent controls on possession and supply. Gabapentin closely resembles pregabalin, a schedule V drug under the Controlled Substances Act in its chemical structure and pharmacological activity. The chemical structure of gabapentin is derived from the addition of a lipophilic cyclohexyl group to the backbone of GABA. Amendment adding drug products in finished dosage formulation that has been approved by the U.S. Food and Drug Administration that contains cannabidiol (2-[1R-3-methyl-6R-(1-methylethenyl)-2-cyclohexen-1-yl]-5-pentyl-1,3-benzenediol) derived from cannabis and no more than 0.1 percent (w/w) residual tetrahydrocannabinols to Schedule V Schedule IV drugs, substances, or chemicals are defined as drugs with a low potential for abuse and low risk of dependence. Some examples of Schedule IV drugs are: Xanax, Soma, Darvon, Darvocet, Valium, Ativan, Talwin, Ambien, Tramadol. Schedule IV - The drug or other substance has a low potential for abuse relative to the drugs or other substances in schedule III. The drug or other substance has a currently accepted medical use in treatment in the United States. IV Schedule IV V Schedule V ExRx Excepted* ExAS Excepted Anabolic Steroid* Drug Measures GM Gram HR Hour MCG Microgram MG Milligram ML Milliliter Miscellaneous APAP Acetaminophen HCL Hydrochloride N/A Not Applicable # Number C.S.A. Controlled Substance Act *These are drug products which: (1) may be dispensed only upon a prescription issued by a Following concerns about abuse, gabapentin has been reclassified as a Class C controlled substance and is now a Schedule 3 drug, but is exempt from safe custody requirements. Healthcare professionals should evaluate patients carefully for a history of drug abuse before prescribing gabapentin, and observe patients for signs of abuse and dependence. Is Gabapentin a Schedule 4 drug? Yes, gabapentin is classified as a Schedule V controlled substance in some states, while it is controlled federally in all states due to its similarity to pregabalin, which is a Schedule V drug. Some states classify it as a Schedule V controlled substance due to concerns about misuse and its involvement in the opioid crisis. Others do not schedule it but require mandatory reporting to state prescription drug monitoring programs (PDMPs) to track prescribing and dispensing. • Schedule V drugs have an even lower potential for abuse, but may include a limited quantity of narcotics. Examples include some cough medicines (Robitussin AC) and Lyrica. WHAT DRUGS DO PDMPs MONITOR? PDMPs track Schedule II, III and IV drugs in every state, and Schedule V drugs in 33 states and D.C., including Pennsylvania. The drug is a sample of a schedule IV or schedule V controlled substance that is provided to the patient without charge. In order to be exempt from reporting, a sample of a Schedule IV drug must be in a quantity limited to an amount that is adequate to treat a patient for a maximum of seventy-two (72) hours. Instalments and repeatable prescriptions. Prescriptions for Schedule 2 or 3 Controlled Drugs can be dispensed by instalments. An instalment prescription must have an instalment direction including both the dose and the instalment amount specified separately on the prescription, and it must also state the interval between each time the medicine can be supplied.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |